786593-06-4
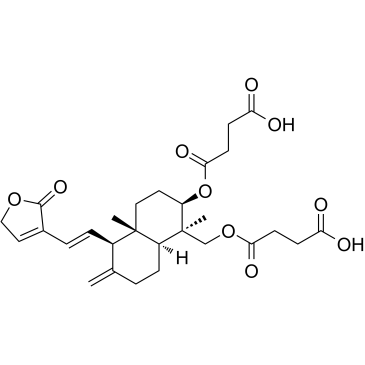
| Name | 4-[[(1R,2R,4aS,5R,8aS)-2-(3-carboxypropanoyloxy)-1,4a-dimethyl-6-methylidene-5-[(2E)-2-(2-oxofuran-3-ylidene)ethyl]-3,4,5,7,8,8a-hexahydro-2H-naphthalen-1-yl]methoxy]-4-oxobutanoic acid |
|---|---|
| Synonyms |
UNII-0X50BP49M1
Butanedioic acid, mono[[(1R,2R,4aR,5R,8aS)-2-(3-carboxy-1-oxopropoxy)-5-[(E)-2-(2,5-dihydro-2-oxo-3-furanyl)ethenyl]decahydro-1,4a-dimethyl-6-methylene-1-naphthalenyl]methyl] ester 4-({(1R,2R,4aR,5R,8aS)-2-[(3-Carboxypropanoyl)oxy]-1,4a-dimethyl-6-methylene-5-[(E)-2-(2-oxo-2,5-dihydro-3-furanyl)vinyl]decahydro-1-naphthalenyl}methoxy)-4-oxobutanoic acid Dehydroandrographolide succinate 4-({(1R,2R,4aR,5R,8aS)-2-[(3-Carboxypropanoyl)oxy]-1,4a-dimethyl-6-methylene-5-[(E)-2-(2-oxo-2,5-dihydrofuran-3-yl)vinyl]decahydronaphthalen-1-yl}methoxy)-4-oxobutanoic acid |
| Description | Dehydroandrographolide succinate, extracted from herbal medicine Andrographis paniculata (Burm f) Nees, is widely used for the treatment of viral pneumonia and viral upper respiratory tract infections because of its immunostimulatory, anti-infective and anti-inflammatory effect[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 733.8±60.0 °C at 760 mmHg |
| Melting Point | 136-138ºC |
| Molecular Formula | C28H36O10 |
| Molecular Weight | 532.579 |
| Flash Point | 239.1±26.4 °C |
| Exact Mass | 532.230835 |
| PSA | 153.50000 |
| LogP | 2.85 |
| Vapour Pressure | 0.0±5.2 mmHg at 25°C |
| Index of Refraction | 1.562 |
| Hazard Codes | Xi |
|---|
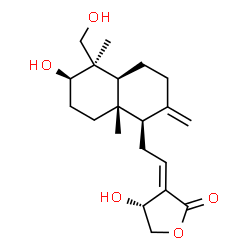
| Precursor 1 | |
|---|---|
| DownStream 0 | |