4394-00-7
| Name | Niflumic acid |
|---|---|
| Synonyms |
EINECS 224-516-2
MFCD00010569 2-(3-Trifluoromethyl-phenylamino)-; nicotinic acid Niflumic acid 2-(a,a,a-Trifluoro-m-toluidino)nicotinic Acid 2-{[3-(Trifluoromethyl)phenyl]amino}nicotinic acid 2-[[3-(Trifluoromethyl)phenyl]amino]-3-pyridinecarboxylic Acid 2-(3-TrifluoroMethylanilino)nicotinic Acid 2-((3-(Trifluoromethyl)phenyl)amino)nicotinic acid 2-[3-(trifluoromethyl)anilino]pyridine-3-carboxylic acid 2-[(3-Trifluoromethylphenyl)amino]nicotinic Acid |
| Description | Niflumic acid, a Ca2+-activated Cl- channel blocker, is an analgesic and anti-inflammatory agent used in the treatment of rheumatoid arthritis.Target: Othersniflumic acid, an inhibitor of calcium-activated chloride currents. Niflumic acid does not block directly calcium channels or activate potassium channels. Niflumic acid selectively reduces a component of noradrenaline- and 5-HT-induced pressor responses by inhibiting a mechanism which leads to the opening of voltage-gated calcium channels [1]. Niflumic acid molecule is completely buried in the substrate-binding hydrophobic channel. The conformations of the binding site in PLA(2) as well as that of niflumic acid are not altered upon binding [2]. Niflumic acid (NFA) produces biphasic behavior on human CLC-K channels that suggests the presence of two functionally different binding sites: an activating site and a blocking site [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 378.0±42.0 °C at 760 mmHg |
| Melting Point | 203-204ºC |
| Molecular Formula | C13H9F3N2O2 |
| Molecular Weight | 282.218 |
| Flash Point | 182.4±27.9 °C |
| Exact Mass | 282.061615 |
| PSA | 62.22000 |
| LogP | 4.94 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Storage condition | Store at RT |
| Water Solubility | Soluble in ethanol (~50 mg/ml), acetone (50 mg/ml, Clear to Slightly Hazy, Yellow), methanol (~50 mg/ml), DMSO (56 mg/ml at 25°C), and acetonitrile (~50 mg/ml). |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H315-H319-H335 |
| Precautionary Statements | P261-P280-P301 + P312 + P330-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | QT2999100 |
| Packaging Group | Ⅲ |
| HS Code | 2933399090 |
| Precursor 6 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
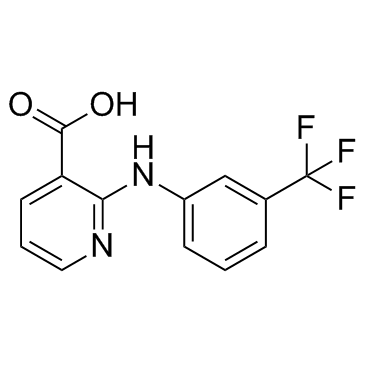
![1-(3'-trifluoromethylphenyl)-2-methyl-4H-1,2-dihydro-pyrido-[2,3-d]-[1,3]-oxazin-4-one structure](https://image.chemsrc.com/caspic/495/137488-50-7.png)
![1-(3'-trifluoromethylphenyl)-2-chloromethyl-4H-1,2-dihydro-pyrido-[2,3-d]-[1,3]-oxazin-4-one structure](https://image.chemsrc.com/caspic/350/137488-49-4.png)
![1-(3'-trifluoromethylphenyl)-2-ethoxy-4H-1,2-dihydro-pyrido-[2,3-d]-[1,3]-oxazin-4-one structure](https://image.chemsrc.com/caspic/388/137488-48-3.png)
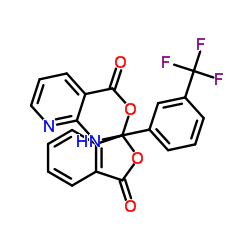
![2-[3-(trifluoromethyl)anilino]pyridine-3-carbonyl chloride structure](https://image.chemsrc.com/caspic/307/70458-49-0.png)