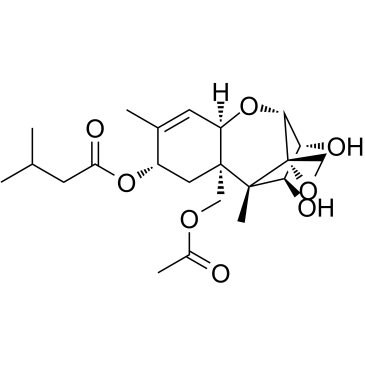
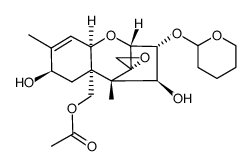
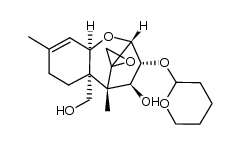
26934-87-2
| Name | ht-2 toxin |
|---|---|
| Synonyms |
HT-2
T-2,Toxin analog Butanoic acid, 3-methyl-, (2α,3α,4β,6β,8β,11β,12α)-15-(acetyloxy)-12,13-epoxy-3,4-dihydroxytrichothec-9-en-8-yl ester valerate Toxin HT 2 (3β,4α,8α)-15-Acetoxy-3,4-dihydroxy-12,13-epoxytrichothec-9-en-8-yl 3-methylbutanoate (3α,4β,8α)-15-(acetyloxy)-3,4-dihydroxy-12,13-epoxytrichothec-9-en-8-yl 3-methylbutanoate Pentanoic acid anion Trichothec-9-ene-3α,4β,8α,15-tetrol, 12,13-epoxy-, 15-acetate 8-isovalerate (8CI) Mycotoxin HT 2 12,13-Epoxytrichothec-9-ene-3-α,4-β,8-α,15-tetrol 15-acetate 8-isovalerate ht-2toxin solution |
| Description | HT-2 Toxin is an active, deacetylated metabolite of the T-2 toxin. HT-2 toxin inhibits protein synthesis and cell proliferation in plants[1][2]. |
|---|---|
| Related Catalog | |
| References |
[2]. Berthiller, F., et al. Masked mycotoxins: A review Mol.Nutr.Food Res. 57, 165-186 (2013). |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 537.1±50.0 °C at 760 mmHg |
| Molecular Formula | C22H32O8 |
| Molecular Weight | 424.485 |
| Flash Point | 179.8±23.6 °C |
| Exact Mass | 424.209717 |
| PSA | 114.82000 |
| LogP | 2.27 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.562 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | T+,T,Xn,F |
|---|---|
| Risk Phrases | 26/27/28-36/37/38-36-20/21/22-11 |
| Safety Phrases | 22-26-36/37/39-45-36/37-16 |
| RIDADR | UN 3462 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS | YD0050000 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| Precursor 3 | |
|---|---|
| DownStream 0 | |