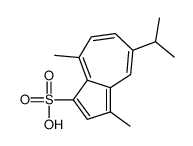
6223-35-4
| Name | sodium,3,8-dimethyl-5-propan-2-ylazulene-1-sulfonate |
|---|---|
| Synonyms |
sodium 5-isopropyl-3,8-dimethylazulene-1-sulfonate
Gualenate de sodium sodium gualenate Azulene sulfonate sodium 5-isopropyl-3,8-dimethyl-azulene-1-sulfonic acid,sodium-salt 5-Isopropyl-3,8-dimethyl-azulen-1-sulfonsaeure,Natrium-Salz Natrii gualenas Sodium guiazulene sulfonate Gualenato sodidico Azulen SN Azupromin |
| Description | Sodium gualenate (Guaiazulenesulfonate sodium) is a hydrophilic derivative of guaiazulene with excellent anti-inflammatory and wound-healing effects mainly used for the treatment of duodenal ulcer, gastric ulcer and gastritis. |
|---|---|
| Related Catalog | |
| In Vitro | Sodium gualenate is an unstable compound, which is gradually decomposed in the solid state at room temperature. When heated, Sodium gualenate decomposes almost completely within 1 week. It was found that a kneaded mixture of Sodium gualenate and cornstarch (weight ratio; 1:250) for tableting with water is stable. So, during production, Sodium gualenate could be stabilized using water[1]. Sodium gualenate slightly inhibits the histamine release from rat peritoneal mast cells and strongly inhibits the leukocyte emigration induced by fMLP[2]. |
| In Vivo | Sodium gualenate has been frequently used for the treatment of human gastritis. Cytoprotection is defined as the main mechanism of Sodium gualenate to protect the mucosa of the stomach and the antipeptic actions in vivo have also been shown[2]. |
| References |
| Melting Point | 98°C(lit.) |
|---|---|
| Molecular Formula | C15H17NaO3S |
| Molecular Weight | 300.348 |
| Exact Mass | 300.079620 |
| PSA | 65.58000 |
| LogP | 4.51650 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RTECS | CO4826000 |
|---|---|
| HS Code | 2904100000 |
|
~% 
6223-35-4 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 21, # 19 p. 5722 - 5725 |
|
~% 
6223-35-4 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 21, # 19 p. 5722 - 5725 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2904100000 |
|---|---|
| Summary | 2904100000 derivatives containing only sulpho groups, their salts and ethyl esters。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |