5108-96-3
| Name | Ammonium 1-pyrrolidinedithiocarbamate |
|---|---|
| Synonyms |
1-Pyrrolidinecarbodithioic acid,ammonium salt
EINECS 225-834-4 Pyrrolindinedithiocarbamate ammonium 1-Pyrrolidinecarbodithioic acid ammoniate (1:1) Ammonium Pyrrolidine-N-dithiocarbamate ammonium tetramethylenedithiocarbamate Pyrrolidine-N-dithiocarbamic Acid Ammonium Salt Ammonium 1-pyrrolidinedithiocarboxylate APDTC AMMONIUM SALT Ammonium pyrrolidinecarbodithioate MFCD00012720 APCD PDTC,NH4 PYRROLIDINEDITHIOCARBAMATE APDTC 1-PYRROLIDINECARBODITHIOIC ACID AMMONIUM SALT QX 314 chloride pyrrolidine-1-dithiocarboxylic acid ammonium salt Ammonium pyrrolidine 1-Pyrrolidinecarbodithioic acid, ammonuim salt Ammonium pyrrolidine dithiocarbamate Pyrrolidinedithiocarbamic acid ammonium salt APDC Ammonium 1-Pyrrolidinecarbodithioate 1-PYRROLIDINECARBODITHIOIC ACID Pyrrolidinedithiocarbamate ammonium APDC AMMONIUM SALT Ammonium 1-pyrrolidinedithiocarbamate Ammoniumpyrrolidinedithiocarb Ammonium pyrrolidine-1-carbodithioate 1-Pyrrolidinecarbodithioic acid, ammonium salt 1-Pyrrolidinecarbodithioic acid ammonium salt,APDC,Ammonium pyrrolidinecarbodithioate Ammonium pyrrolidinedithiocarbamate Pyrrolidinedithiocarbamate (ammonium) |
| Description | Pyrrolidinedithiocarbamate ammonium is a selective NF-κB inhibitor. |
|---|---|
| Related Catalog | |
| Target |
NF-κB |
| In Vitro | Pretreatment of cells with pyrrolidinedithiocarbamate (3-1000 μM) dose-dependently attenuate IL-8 production. Furthermore, pyrrolidinedithiocarbamate (100 μM) suppresses the accumulation of IL-8 mRNA. Pyrrolidinedithiocarbamate inhibits the activation of NF-κB, because pyrrolidinedithiocarbamate suppresses both NF-κB DNA binding and NF-κB-dependent transcriptional activity. NF-κB inhibition with pyrrolidinedithiocarbamate decrease IL-8 production by intestinal epithelial cells[1]. |
| In Vivo | The DSS+pyrrolidinedithiocarbamate ammonium-treated groupII exhibits suppression of shortening of intestinal length and reduction of DAI score. Activated NF-κB level and IL-1β and TNF-α levels are significantly lower in DSS+pyrrolidinedithiocarbamate ammonium-treated groupII. These findings suggest that suppression of NF-κB activity by pyrrolidinedithiocarbamate ammonium can delay the healing of mucosal tissue defects (erosions or ulcers) arising from inflammation, but that it can strongly suppress the expression of inf-lammatory cytokines (IL-1β and TNF-α), resulting in significant alleviation of colitis. pyrrolidinedithiocarbamate ammonium is useful for the treatment of ulcerative colitis[2]. |
| Cell Assay | The human colon cancer cell line HT-29 is obtained and cells are grown in modified McCoy’s 5A medium supplemented with 10% fetal bovine serum. To study the effect of pyrrolidinedithiocarbamate ammonium on IL-8 production, HT-29 cells in 96-well plates are induced with 20 ng/mL of IL-1β for 18 h. Various concentrations (3-1000 μM) of pyrrolidinedithiocarbamate or its vehicle (culture medium) are added to the cells 30 min prior to IL-1β stimulation. The concentration of IL-8 in the supernatant is determined using solid-phase enzyme-linked immunosorbent assay[1]. |
| Animal Admin | Animal Administration: [2]Pyrrolidinedithiocarbamate is administered intraperitoneally to mice at dose levels of 100 and 50 mg/kg. Mice are divided into a DSS-untreated group (normal group), DSS-treated control group, DSS+pyrrolidinedithiocarbamate-treated groupI (low-dose group), and DSS+pyrrolidinedithiocarbamate-treated groupII (high-dose group). In each group, the disease activity index score (DAI score), intestinal length, histological score, and the levels of activated NF-κB and inflammatory cytokines (IL-1β and TNF-α) in tissue are measured[2]. |
| References |
| Density | 1.264g/cm3 |
|---|---|
| Boiling Point | 199.7ºC at 760 mmHg |
| Melting Point | 153-155 °C(lit.) |
| Molecular Formula | C5H12N2S2 |
| Molecular Weight | 164.292 |
| Flash Point | 74.6ºC |
| Exact Mass | 164.044189 |
| PSA | 77.37000 |
| LogP | 1.55870 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | 50 g/L (20 ºC) |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29339990 |
|
~85% 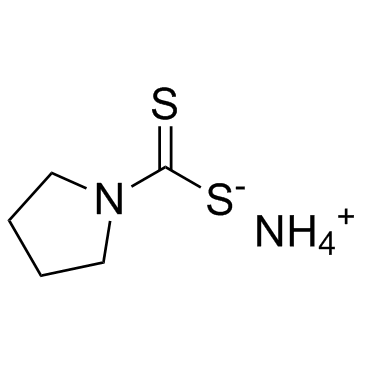
5108-96-3 |
| Literature: Menezes; Vieira; De Lima; Porto; Cortes; Ardisson; Albrecht-Schmitt European Journal of Medicinal Chemistry, 2005 , vol. 40, # 12 p. 1277 - 1282 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |