41859-67-0
| Name | Bezafibrate |
|---|---|
| Synonyms |
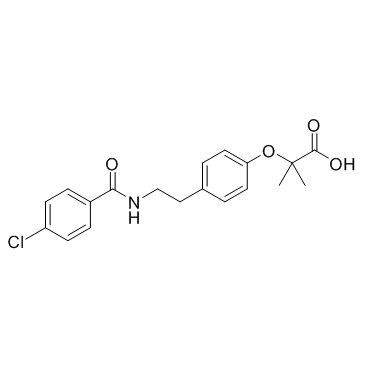
a-[4-(4-Chlorobenzoylaminoethyl)phenoxy]isobutyric Acid
Difaterol bezafibric acid bezafibrato bezafibratum [INN_la] 2-[4-[2-[(4-Chlorobenzoyl)amino]ethyl]phenoxy]-2-methylpropanoic Acid 2-[4-[2-(4-Chlorobenzamido)ethyl]phenoxy]-2-methylpropanoic acid 2-(4-{2-[(4-Chlorobenzoyl)amino]ethyl}phenoxy)-2-methylpropanoic acid CEDUR Bezalip MFCD00078970 Bezafibrate 2-[p-[2-(p-Chlorobenzamido)ethyl]phenoxy]-2-methylpropionic Acid 2-[4-[2-(4-Chlorobenzamido)ethyl]phenoxy]isobutyric Acid Befizal bezafibrat Bezatol bf-759 EINECS 255-567-9 |
| Description | Bezafibrate is an agonist of PPAR, with EC50s of 50 μM, 60 μM, 20 μM for human PPARα, PPARγ and PPARδ, and 90 μM, 55 μM, 110 μM for murine PPARα, PPARγ and PPARδ, respectively; Bezafibrate is used as an hypolipidemic agent. |
|---|---|
| Related Catalog | |
| Target |
hPPARδ:20 μM (EC50) hPPARα:50 μM (EC50) hPPARγ:60 μM (EC50) |
| In Vitro | Bezafibrate is an agonist of PPAR, with EC50s of 90 μM, 55 μM, 110 μM for murine PPARα, PPARγ and PPARδ, and 50 μM, 60 μM, 20 μM for human PPARα, PPARγ and PPARδ, respectively[1]. Bezafibrate (> 200 μM) shows significant cytotoxicity against human retinal microvascular endothelial cells (HRMECs) and human retinal pigment epithelial ARPE-19 cells. Bezafibrate (30-100 μM) suppresses tumor necrosis factor (TNF)α induced inflammatory factors and regulates TNFα induced nuclear factor (NF)-κB transactivation in HRMEC. Bezafibrate inhibits VEGF-induced HRMECs migration, and inhibits interleukin (IL)-1β-induced VEGF secretion of ARPE-19 cells[2]. |
| In Vivo | Bezafibrate (0.5%) markedly reduces plasma lipid and glucose levels, and increases islet area in the pancreas in TallyHo mice. Bezafibrate also improves energy expenditure and metabolic flexibility. Moreover, Bezafibrate ameliorates steatosis, modifies lipid composition and increases mitochondrial mass in the liver[3]. |
| Cell Assay | Cell viability is assessed using the CCK-8 kit. Hhuman retinal microvascular endothelial cells (HRMECs) or ARPE-19 cells are seeded at 5000 cells/well in medium containing 10% serum in 96-well plates. After a 24-h incubation, the medium is serum-starved with 1% FBS for 6 h, the CCK-8 reagent is added, and the absorbance of the resultant solution is measured at 450 nm by using a microplate reader at three time points, 24, 48, and 72 h after treatment with Bezafibrate (0, 10, 50, 100, 200, 500, and 1000 μM)[2]. |
| Animal Admin | TallyHo mice are bred in our animal facility. Only male mice are used in the study, and mice receive a standard diet (SD), which is supplemented with 0.5% (w/w) Bezafibrate for the Bezafibrate groups for 8 weeks. Animals are killed by isoflurane overdose, and dissected tissues are prepared as stated below. All data represent samples taken after 8 weeks of Bezafibrate (or SD) treatment unless otherwise stated[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 572.1±45.0 °C at 760 mmHg |
| Melting Point | 184 °C |
| Molecular Formula | C19H20ClNO4 |
| Molecular Weight | 361.819 |
| Flash Point | 299.8±28.7 °C |
| Exact Mass | 361.108093 |
| PSA | 75.63000 |
| LogP | 3.46 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.583 |
| Storage condition | Refrigerator |
| Water Solubility | DMF: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | UE8755000 |
| HS Code | 2924299090 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |