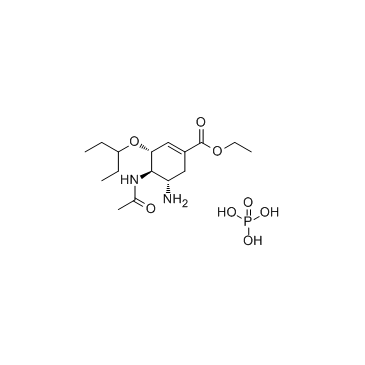
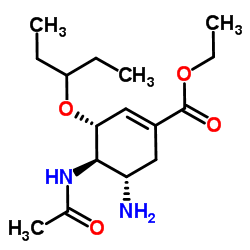
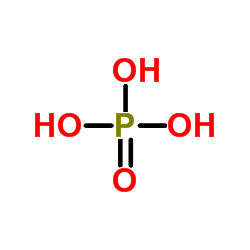
204255-11-8
| Name | oseltamivir phosphate |
|---|---|
| Synonyms |
Ro-64-0796/002
Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(3-pentanyloxy)-1-cyclohexene-1-carboxylate phosphate (1:1) Oseltamivir phosphate Tamiflu Oselt MFCD08059548 Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate phosphate (1:1) GS-4104/002 Osteltamivir phosphate ethyl (3R,4R,5S)-4-(acetylamino)-5-amino-3-(1-ethylpropoxy)cyclohex-1-ene-1-carboxylate phosphate ethyl (3R,4R,5S)-4-(acetylamino)-5-amino-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate phosphate (1:1) Phosphorosäure-ethyl-(3R,4R,5S)-4-(acetylamino)-5-amino-3-(1-ethylpropoxy)cyclohex-1-en-1-carboxylat(1:1) Ro 64-0796/002 NTERMEDIATES OF OSELTAMIVIR 1-Cyclohexene-1-carboxylic acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, ethyl ester, (3R,4R,5S)-, phosphate (1:1) acide phosphorique - (3R,4R,5S)-4-(acétylamino)-5-amino-3-(1-éthylpropoxy)cyclohex-1-ène-1-carboxylate d'éthyle (1:1) OseltaMivir Acid-D3 Phosphate Oseltamivir phosphat Oseltamir phosphate Oseltamivir (phosphate) |
| Description | Oseltamivir phosphate (GS 4104) is a neuraminidase inhibitor recommended for the treatment and prophylaxis of influenza A and B. |
|---|---|
| Related Catalog | |
| Target |
Influenza A and B[1] |
| In Vitro | Oseltamivir phosphate (OP) is a prodrug that is readily absorbed from the gastrointestinal tract after oral administration and is extensively converted predominantly by hepatic esterases to Oseltamivir carboxylate (OC)[1]. Oseltamivir phosphate is a widely used anti-influenza sialidase inhibitor. The metabolic activity of CMA07 and CMT-U27 cell lines is significantly decreased with 305 μM Oseltamivir phosphate treatment (p=0.005 and p<0.0001 respectively) using One Way ANOVA testes. In contrast, no statistically significant alterations are observed with 0.305 μM (p=0.9781), 3.05 μM (p=0.7436) and 30.5 μM (p=0.9623) of Oseltamivir phosphate treatments when compare with control cells. Finally, to assess the effect of Oseltamivir phosphate on CMA07 and CMT-U27 programmed cell death, and given that 305 μM Oseltamivir phosphate treatment impaired cell metabolic activity, a programmed cell death measurement is performed with the TUNEL assay. Twenty-four hour Oseltamivir phosphate treatment, specifically at 305 μM, significantly increases CMA07 (p=0.001) and CMT-U27 (p=0.0002) DNA fragmentation, suggesting promotion of programmed cell death, when compare with lower Oseltamivir concentrations, or with PBS[2]. |
| In Vivo | Oseltamivir phosphate-treated mice present significantly more inflammatory infiltrate in primary tumors (p=0.01). Ki-67 antigen and caspase-3 protein are used to assess CMT-U27 xenograft tumor cell proliferation and apoptosis respectively. Virtually no differences are found in Ki-67 and caspase 3 (p=0.2) expression between Oseltamivir-treated and non-treated mice[2]. |
| Cell Assay | CMA07 and CMT-U27 cells are cultured in 24-well plates in triplicate for each condition: 0.305 μM, 3.05 μM, 30.5 μM and 305 μM Oseltamivir phosphate and PBS is used as control. Cells are counted every day for 7 days in a Neubauer’s chamber in a 1:2 dilution of cells in 0.4% trypan blue and cell count is done using the volume conversion factor for 1 mm3, which is 1×104. This assay is repeated 3 times and growth curves are traced[2]. |
| Animal Admin | Mice[2] Female NIH(S)II-nu/nu nude mice, aged 4-6 weeks, are orthotopically inoculated with 1×106 viable CMT-U27 canine breast cancer cells in the mammary fat pad using a 25 gauge needle. A total of 8 mice are inoculated. When nodules reached a volume of approximately 500 mm3, mice (n=8) are randomized and divided into control group (n=4) and treatment group (n=4).The animals receive intraperitoneally (IP) dailly either 100 μL of PBS (control group) or 100mg/Kg of Oseltamivir phosphate, diluted in PBS (treatment group) until time of death. Tumor size is measured using calipers, and tumor volume (mm3) is estimated by width×length×height. |
| References |
| Density | 1.08g/cm3 |
|---|---|
| Boiling Point | 473.3ºC at 760 mmHg |
| Melting Point | 196-198°C |
| Molecular Formula | C16H31N2O8P |
| Molecular Weight | 410.400 |
| Flash Point | 240ºC |
| Exact Mass | 410.181793 |
| PSA | 178.22000 |
| LogP | 1.44800 |
| Storage condition | -20°C Freezer |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Oseltamivir phosphate Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed. Section3. Composition/information on ingredients. Ingredient name:Oseltamivir phosphate CAS number:204255-11-8 Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Storage:Store in closed vessels, under −20◦C. Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified Boiling point:No data Melting point:No data Flash point:No data Density:No data Molecular formula: C16H28N2O4.H3O4P Molecular weight: 410.4 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | Xn |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2942000000 |
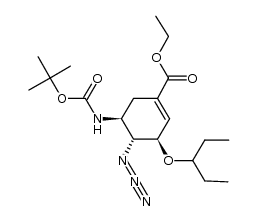
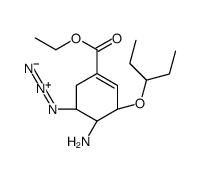
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |


![(1S,5R,6S)-Ethyl 5-(pentan-3-yl-oxy)-7-oxa-bicyclo[4.1.0]hept-3-ene-3-carboxylate structure](https://image.chemsrc.com/caspic/152/204254-96-6.png)

![ETHYL (1R,5R,6R)-5-(1-ETHYLPROPOXY)-7-AZABICYCLO[4.1.0]HEPT-3-ENE-3-CARBOXYLATE structure](https://image.chemsrc.com/caspic/341/204255-02-7.png)
![1,3-Benzodioxole-5-carboxylic acid, 3a,6,7,7a-tetrahydro-2,2-dimethyl-7-[(Methylsulfonyl)oxy]-, ethyl ester, (3aR,7R,7aR) structure](https://image.chemsrc.com/caspic/435/204254-84-2.png)