67-99-2
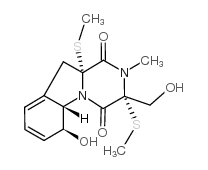
| Name | gliotoxin |
|---|---|
| Synonyms |
(1R,7S,8S,11R)-7-Hydroxy-11-(hydroxymethyl)-15-methyl-12,13-dithia-9,15-diazatetracyclo[9.2.2.0.0]pentadeca-3,5-diene-10,14-dione
epithiodiketopiperazine Gliotoxin,(3R,5aS,6S,10aR)-2,3,5a,6-Tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-10H-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione MFCD00058534 (3R,5aS,6S,10aR)-6-hydroxy-3-(hydroxymethyl)-2-methyl-2,3,6,10-tetrahydro-5aH-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione [3R-(3a,5ab,6b,10aa)]-2,3,5a,6-Tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-10H3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione Gliotoxin |
| Description | Gliotoxin is a secondary metabolite, the most abundant mycotoxin secreted by A. fumigatus, inhibits the phagocytosis of macrophages and the immune functions of other immune cells [1]. Gliotoxin inhibits inducible NF-κB activity by preventing IκB degradation, which consequently induces host-cell apoptosis[2]. Gliotoxin activates PKA and increases intracellular cAMP concentration; modulates actin cytoskeleton rearrangement to facilitate A. fumigatus internalization into lung epithelial cells[3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 699.7±55.0 °C at 760 mmHg |
| Melting Point | 153.5ºC |
| Molecular Formula | C13H14N2O4S2 |
| Molecular Weight | 326.391 |
| Flash Point | 377.0±31.5 °C |
| Exact Mass | 326.039490 |
| PSA | 131.68000 |
| LogP | 0.52 |
| Vapour Pressure | 0.0±5.0 mmHg at 25°C |
| Index of Refraction | 1.814 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302-H312-H319-H332 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T |
| Risk Phrases | 25 |
| Safety Phrases | S45;S36/S37/S39 |
| RIDADR | UN 3462 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | KB4725000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29419090 |
| Precursor 2 | |
|---|---|
| DownStream 2 | |


![(3aS,5S,6aR,7S,9aR)-6-tert-butyl 5-methyl 2-thioxo-7-((triisopropylsilyl)oxy)-4,5,6a,7-tetrahydro-[1,3]dioxolo[4,5-d]indole-5,6(9aH)-dicarboxylate structure](https://image.chemsrc.com/caspic/135/1402592-69-1.png)
![Pyrazino[1,2-a]indole-1,4-dione,2,3-dihydro-2-methyl-3-methylene structure](https://image.chemsrc.com/caspic/271/19079-11-9.png)