445479-97-0
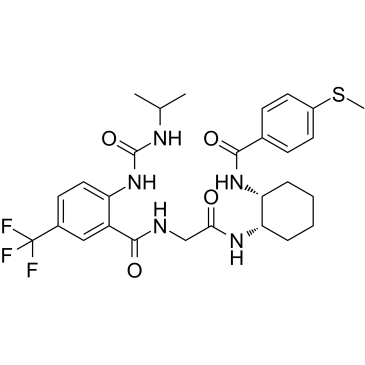
| Name | N-[2-[[(1R,2S)-2-[(4-methylsulfanylbenzoyl)amino]cyclohexyl]amino]-2-oxoethyl]-2-(propan-2-ylcarbamoylamino)-5-(trifluoromethyl)benzamide |
|---|---|
| Synonyms | Carboxy-PTIO,potassium salt |
| Description | BMS CCR2 22 is a potent, specific and high affinity CC-type chemokine receptor 2 (CCR2) antagonist with excellent binding affinity (binding IC50 of 5.1 nM) and potent functional antagonism (calcium flux IC50 of 18 nM and chemotaxis IC50 of 1 nM)[1][2]. |
|---|---|
| Related Catalog | |
| Target |
CCR2:5.1 nM (IC50) |
| In Vitro | BMS CCR2 22 (Compound 22) has binding affinity for wild-type and E291A mutants with IC50 values of 7.5 nM and 3.7 nM, respectively[1].BMS CCR2 22 prevents both the binding and the internalization of fluorescently labeled hMCP-1_AF647 internalization in human monocytes. BMS CCR2 22 inhibits the internalization of hMCP1_AF647 with an IC50 value of approximately 2 nM[2]. The addition of BMS CCR2 22 (0.1-10 μM; 24 h), cenicriviroc (CVC) or a combination of both BMS CCR2 22 and MVC to human aortic endothelial cells (HAoECs) prior to MCP-1 stimulation do not alter E-selectin, ICAM-1, or CD99 cell surface expression. Incubation of HAoECs with BMS CCR2 22 before MCP-1 significantly increases VCAM-1 and PECAM1 cell surface levels (from 72.8 to 160% and from 97.2 and 127%, respectively)[3]. |
| References |
| Molecular Formula | C28H34F3N5O4S |
|---|---|
| Molecular Weight | 593.66100 |
| Exact Mass | 593.22800 |
| PSA | 153.73000 |
| LogP | 6.18100 |