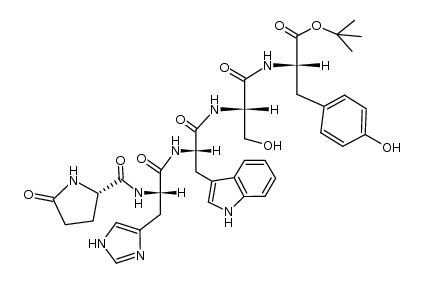
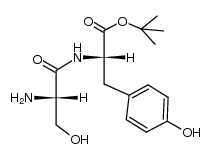
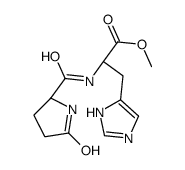
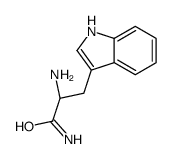
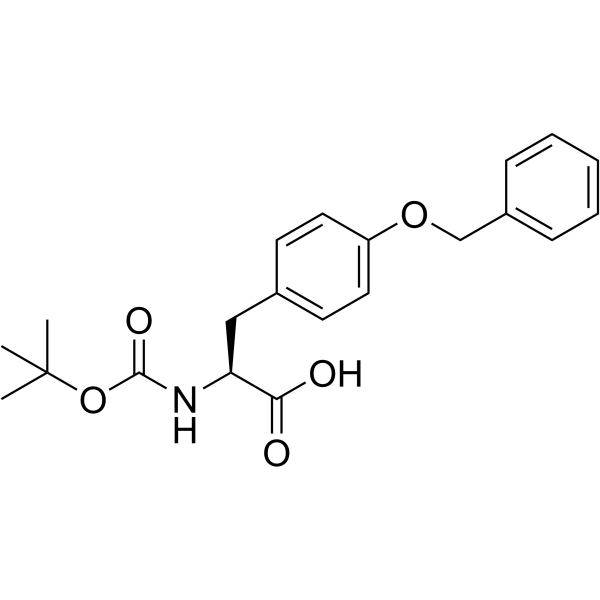
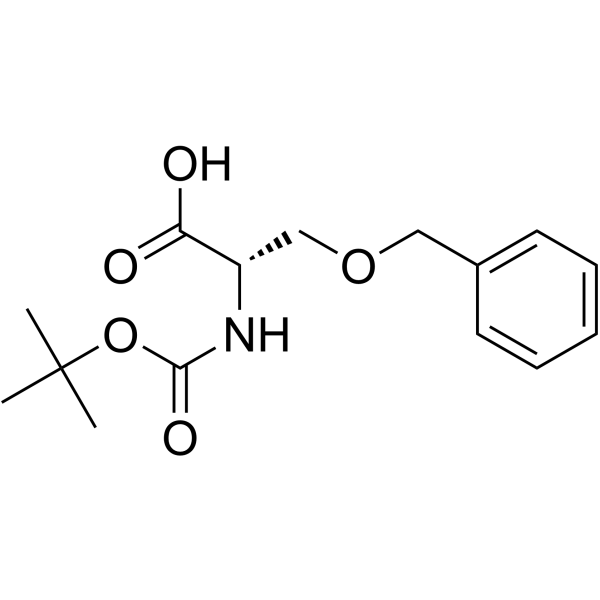
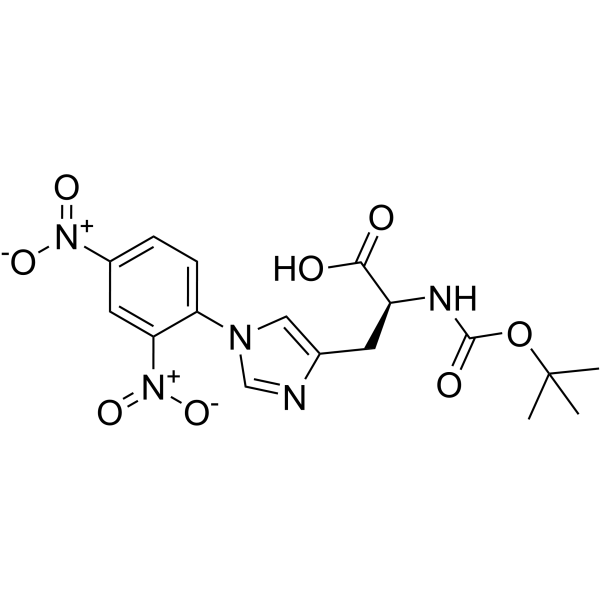
52434-75-0
| Name | lhrh (1-5) |
|---|---|
| Synonyms |
pglu-his-trp-ser-tyr
pyr-his-trp-ser-tyr-oh |
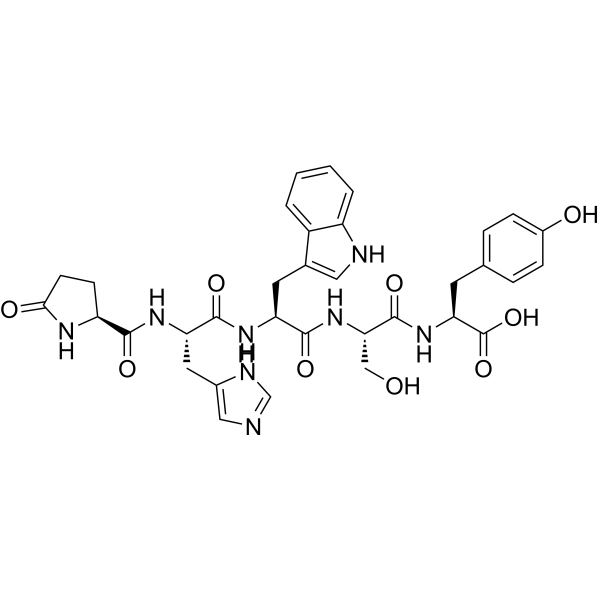
| Description | LHRH (1-5) (free acid) is a polypeptide generated by the cleavage of LHRH at the Tyr55-Gly66 site. LHRH (1-5) (free acid) is converted into LHRH (1-3) and LHRH (4-5) fragments under the catalysis of Angiotensin-converting enzyme (HY-P2983)[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C34H38N8O9 |
|---|---|
| Molecular Weight | 702.71 |
| Exact Mass | 702.27600 |
| PSA | 267.73000 |
| LogP | 0.81160 |
|
~% 
52434-75-0 |
| Literature: Slomczynska, Urszula; Leplawy, Tomasz; Leplawy, Miroslaw, T. Zeitschrift fuer Naturforschung, B: Chemical Sciences, 1992 , vol. 47, # 10 p. 1424 - 1430 |
|
~% 
52434-75-0 |
| Literature: Slomczynska, Urszula; Leplawy, Tomasz; Leplawy, Miroslaw, T. Zeitschrift fuer Naturforschung, B: Chemical Sciences, 1992 , vol. 47, # 10 p. 1424 - 1430 |
|
~% 
52434-75-0 |
| Literature: Slomczynska, Urszula; Leplawy, Tomasz; Leplawy, Miroslaw, T. Zeitschrift fuer Naturforschung, B: Chemical Sciences, 1992 , vol. 47, # 10 p. 1424 - 1430 |
|
~% 
52434-75-0 |
| Literature: Slomczynska, Urszula; Leplawy, Tomasz; Leplawy, Miroslaw, T. Zeitschrift fuer Naturforschung, B: Chemical Sciences, 1992 , vol. 47, # 10 p. 1424 - 1430 |
|
~% 
52434-75-0 |
| Literature: Slomczynska, Urszula; Leplawy, Tomasz; Leplawy, Miroslaw, T. Zeitschrift fuer Naturforschung, B: Chemical Sciences, 1992 , vol. 47, # 10 p. 1424 - 1430 |
|
~% 
52434-75-0 |
| Literature: Slomczynska, Urszula; Leplawy, Tomasz; Leplawy, Miroslaw, T. Zeitschrift fuer Naturforschung, B: Chemical Sciences, 1992 , vol. 47, # 10 p. 1424 - 1430 |
|
~% 
52434-75-0 |
| Literature: Slomczynska, Urszula; Leplawy, Tomasz; Leplawy, Miroslaw, T. Zeitschrift fuer Naturforschung, B: Chemical Sciences, 1992 , vol. 47, # 10 p. 1424 - 1430 |
|
~%
Detail
|
| Literature: Stetler-Stevenson, Mary Alice; Yang, Dai Chang; Lipkowski, Andrew; McCartney, Linda; Peterson, Darryl; Fluoret, George Journal of Medicinal Chemistry, 1981 , vol. 24, # 6 p. 688 - 692 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |