1312473-63-4
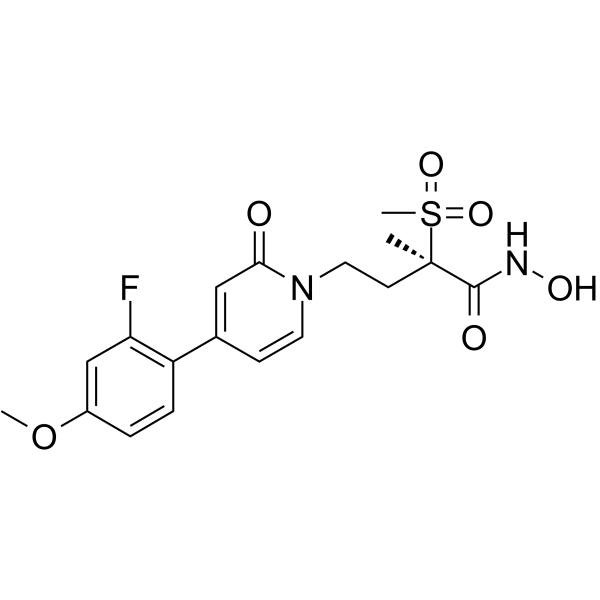
| Name | (2R)-4-[4-(2-fluoro-4-methoxyphenyl)-2-oxopyridin-1-yl]-N-hydroxy-2-methyl-2-methylsulfonylbutanamide |
|---|---|
| Synonyms |
(|AR)-4-(2-Fluoro-4-methoxyphenyl)-N-hydroxy-|A-methyl-|A-(methylsulfonyl)-2-oxo-1(2H)-pyridinebutanamide
MS 245 oxalate |
| Description | PF-5081090 (LpxC-4) is a potent LpxC inhibitor, is a rapidly bactericidal with broad-spectrum activity. PF-5081090 serves as a regulator of lipid A biosynthesis in Gram-negative pathogens[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | PF-5081090 shows strong potency against a broad spectrum of Gram-negative pathogens with IC50s of 1.1 nM (P. aeruginosa), 0.069 nM (K. pneumonia) and MIC90s of 1 μg/mL (P. aeruginosa, K. pneumoniae), 0.25 μg/mL (E. coli), 0.5 μg/mL (Enterobacter spp), 2 μg/mL (S. maltophilia)[1]. PF-5081090 (0.25 μg/mL; 0-50 h) demonstrates sustained bactericidal activities against P. aeruginosa UC12120 (A), PA-1955 (B), and K. pneumoniae KP-1487[1]. PF-5081090 (32 mg/L) increases antibiotic susceptibility in Acinetobacter baumannii with rifampicin, vancomycin, azithromycin, imipenem and amikacin[2]. PF-5081090 (32 mg/L) inhibits lipid A biosynthesis, and significantly increases cell permeability in A. baumannii[2]. |
| In Vivo | PF-5081090 (8.75, 75, 300 mg/kg; s.c.; single dose) exhibits a exposure increasing in linear manner across the dose range in mice, with area under the concentration-time curve (AUC) and maximum concentration of drug in serum (Cmax) increasing with a proportional increase in dose[1]. PF-5081090 shows potent efficacies against sentinel strains of P. aeruginosa and K. pneumonia in CD-1 mice, with effective dose (ED50) ranging from 7.4-55.9 mg/kg (against acute septicemia model), <25 mg/kg (against pneumonia model), and 16.8 mg/kg (against neutropenic thigh model) in mice infected with P. aeruginosa PA-1950[1]. Pharmacokinetics of PF-5081090 in CD-1 micea[1] Dose (mg/kg) Cmax (mg/L) Tmax (h) AUC (h•mg/L) Free AUC (h•mg/L) T1/2 (h) CL (L/h/kg) Vss (L/kg) 18.75 5.02 0.25 5.09 1.58 0.6 3.79 2.20 75 15.50 0.33 17.60 5.46 0.69 4.32 3.30 300 75.40 0.33 76.30 23.70 0.68 3.92 2.53a Following single subcutaneous doses. |
| References |
| Molecular Formula | C18H21FN2O6S |
|---|---|
| Molecular Weight | 412.43300 |
| Exact Mass | 412.11000 |
| PSA | 126.57000 |
| LogP | 3.28290 |
| RIDADR | NONH for all modes of transport |
|---|