15183-37-6
| Name | (8R,9S,13S,14S,15R,16R,17R)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,15,16,17-tetrol |
|---|---|
| Synonyms |
ESTETROL
15|A-Hydroxyestriol |
| Description | Estetrol, a natural estrogen synthesized exclusively during pregnancy by the human fetal liver, is a selective nuclear estrogen receptor modulator. Estetrol exerts estrogenic actions on the endometrium or the central nervous system but presents antagonistic effects on the breast[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Estrogen receptor[1] |
| References |
| Density | 1.343 g/cm3 |
|---|---|
| Boiling Point | 491.9ºC at 760 mmHg |
| Molecular Formula | C18H24O4 |
| Molecular Weight | 304.38100 |
| Flash Point | 231.7ºC |
| Exact Mass | 304.16700 |
| PSA | 80.92000 |
| LogP | 1.55080 |
| Index of Refraction | 1.65 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
15183-37-6 |
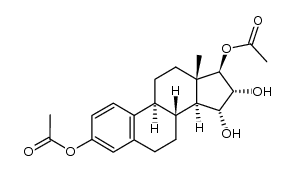
| Literature: Suzuki, Emako; Namba, Susumu; Kurihara, Hiroyuki; Goto, Junichi; Matsuki, Yasuhiko; Nambara, Toshio Steroids, 1995 , vol. 60, # 3 p. 277 - 284 |
|
~% 
15183-37-6 |
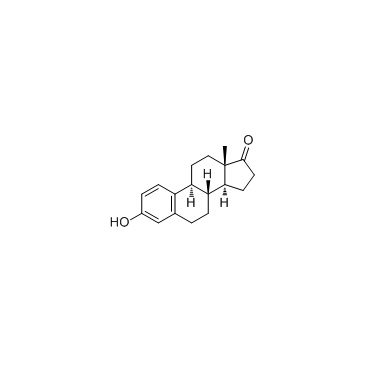
| Literature: EP2383279 A1, ; |
|
~% 
15183-37-6 |
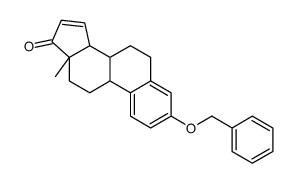
| Literature: EP2383279 A1, ; |
|
~% 
15183-37-6 |
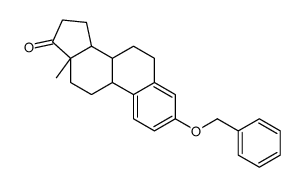
| Literature: EP2383279 A1, ; |
|
~% 
15183-37-6 |
| Literature: EP2383279 A1, ; |
|
~% 
15183-37-6 |
| Literature: Steroids, , vol. 60, # 3 p. 277 - 284 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |