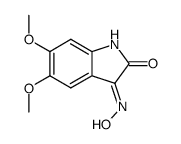
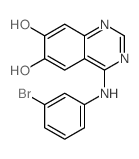
153436-54-5
| Name | N-(3-bromophenyl)-6,7-dimethoxyquinazolin-4-amine |
|---|---|
| Synonyms |
Compound 32
N-(3-bromophenyl)-6,7-dimethoxyquinazolin-4-amine PD-153035 N-(3-Bromophenyl)-6,7-dimethoxy-4-quinazolinamine PD153035 |
| Description | PD153035 (ZM 252868;AG 1517;Tyrphostin AG 1517;SU 5271) is a potent EGFR inhibitor with Ki and IC50 of 6 and 25 pM, respectively. |
|---|---|
| Related Catalog | |
| Target |
EGFR:6 pM (Ki) EGFR:25 pM (IC50) |
| In Vitro | PD153035 inhibits EGF-stimulated receptor autophosphorylation in A431 human epidermoid carcinoma cells, with an IC50 of 14 nM[1]. PD 153035 has little effect on PDGFR, FGFR, CSF-1 receptor, the insulin receptor, or on src tyrosine kinases at concentrations as high as 50 μM. PD 153035 rapidly suppresses autophosphorylation of the EGF receptor at low nanomolar concentrations in fibroblasts or in human epidermoid carcinoma cells and selectively blocks EGF-mediated cellular processes including mitogenesis, early gene expression, and oncogenic transformation[2]. PD153035 causes a dose-dependent growth inhibition of EGF receptor-positive cell lines, beginning at less than micromolar concentrations, and the IC50 is less than 1 pM in most cases[3]. |
| In Vivo | PD153035 levels in the plasma and tumor rise to 50 and 22 μM within 15 minutes following a single i.p. dose of 80 mg/kg. While the plasma levels of PD 153035 falls below 1 μM by 3 hours, in the tumors it remains at micromolar concentrations for at least 12 hours. The tyrosine phosphorylation of the EGF receptor is rapidly suppressed by 80-90% in the tumors[4]. |
| Cell Assay | Different EGF receptor-overexpressing cell lines (A43 1, Difi, MDA-MB-468, MDA-MB-231, DU145, SiHa, C4i, and MEl 80) are treated with PD153035 at increasing concentrations of 0.125-2.5 p.M. Growth inhibitory effect in monolayer cell culture is assessed[3]. |
| Animal Admin | Mice: Mice are injected with PD153035 (80 mg/kg) or vehicle and rumors are excised at 20 minutes and 180 minutes and extracts are prepared. Two mice are used for each time point and the experiment is repeated four times. Within each of the four experiments ANOVA is used to compare the inhibition by PD 153035 of the EGF-stimulation[3]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 472.1±45.0 °C at 760 mmHg |
| Molecular Formula | C16H14BrN3O2 |
| Molecular Weight | 360.205 |
| Flash Point | 239.3±28.7 °C |
| Exact Mass | 359.026947 |
| PSA | 56.27000 |
| LogP | 4.08 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.679 |
| Storage condition | 2-8°C |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933990090 |
|
~90% 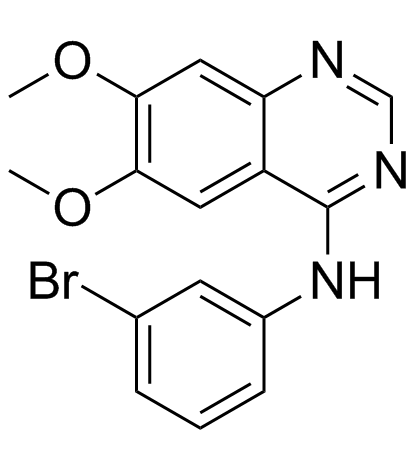
153436-54-5 |
| Literature: Marzaro, Giovanni; Guiotto, Adriano; Pastorini, Giovanni; Chilin, Adriana Tetrahedron, 2010 , vol. 66, # 4 p. 962 - 968 |
|
~89% 
153436-54-5 |
| Literature: Wang, Zheng; Wang, Cuiling; Sun, Yanni; Zhang, Ning; Liu, Zhulan; Liu, Jianli Tetrahedron, 2014 , vol. 70, # 4 p. 906 - 913 |
|
~% 
153436-54-5 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 41, # 7 p. 623 - 629 |
|
~% 
153436-54-5 |
| Literature: Organic Letters, , vol. 6, # 25 p. 4775 - 4778 |
|
~% 
153436-54-5 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 4, # 8 p. 1203 - 1207 |
|
~% 
153436-54-5 |
| Literature: Tetrahedron, , vol. 70, # 4 p. 906 - 913 |
|
~% 
153436-54-5 |
| Literature: Tetrahedron, , vol. 70, # 4 p. 906 - 913 |
|
~% 
153436-54-5 |
| Literature: Tetrahedron, , vol. 70, # 4 p. 906 - 913 |
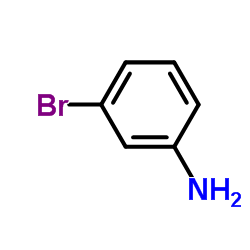
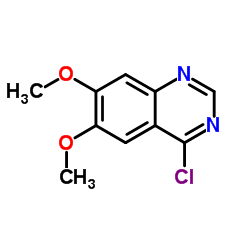
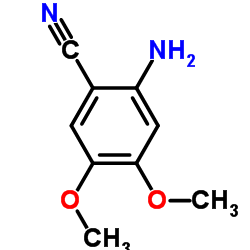
| Precursor 8 | |
|---|---|
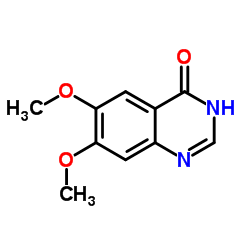
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |