19216-56-9
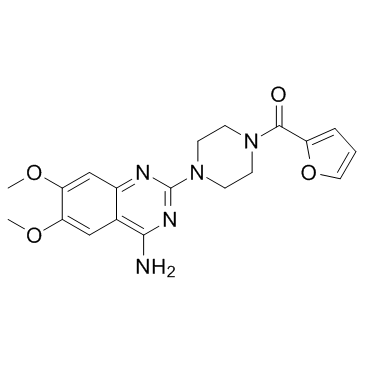
| Name | prazosin |
|---|---|
| Synonyms |
[14C]-Prazosin
Prazosinum [INN-Latin] Minipress (4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl)(furan-2-yl)methanone [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone [125I]-Prazosin Prazosinum 4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazinyl furan-2-yl ketone [Bodipyl-FL]-Prazosin Prazosine Prazosina Furazosin Prazosina [INN-Spanish] 2-[4-(2-furoyl)-piperazin-1-yl]-4-amino-6,7-dimethoxy-quinazoline Prazosine [INN-French] Lentopres |
| Description | Prazosin is an alpha-adrenergic blocker and is a sympatholytic drug used to treat high blood pressure and anxiety, PTSD, and panic disorder.Target: Adrenergic ReceptorPrazosin, is a sympatholytic drug used to treat high blood pressure and anxiety, PTSD, andpanic disorder. It is an alpha-adrenergic blocker that is specific for the alpha-1 receptors. These receptors are found on vascular smooth muscle, where they are responsible for the vasoconstrictive action of norepinephrine. They are also found throughout the central nervous system. As of 2013, prazosin is off-patent in the US, and the FDA has approved at least one generic manufacturer.In addition to its alpha-blocking activity, prazosin is an antagonist of the MT3 receptor (which is not present in humans), with selectivity for this receptor over the MT1 and MT2 receptors.Prazosin is orally active and has a minimal effect on cardiac function due to its alpha-1 receptor selectivity. However, when prazosin is initially started, heart rate and contractility go up in order to maintain the pre-treatment blood pressures because the body has reached homeostasis at its abnormally high blood pressure. The blood pressure lowering effect becomes apparent when prazosin is taken for longer periods of time. The heart rate and contractility go back down over time and blood pressure decreases. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.352g/cm3 |
|---|---|
| Boiling Point | 638.4ºC at 760mmHg |
| Melting Point | 278-280ºC |
| Molecular Formula | C19H21N5O4 |
| Molecular Weight | 383.40100 |
| Flash Point | 339.9ºC |
| Exact Mass | 383.15900 |
| PSA | 107.68000 |
| LogP | 1.71760 |
| Vapour Pressure | 3.4E-16mmHg at 25°C |
| Index of Refraction | 1.651 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2934999090 |
|---|
|
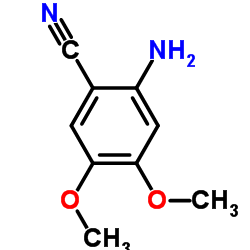
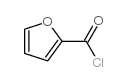
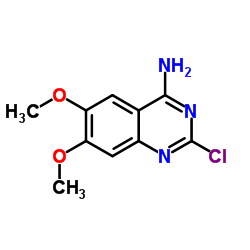
~% 
19216-56-9 |
| Literature: US3935213 A1, ; |
|
~% 
19216-56-9 |
| Literature: US4062844 A1, ; |
|
~% 
19216-56-9 |
| Literature: US4062844 A1, ; |
|
~% 
19216-56-9 |
| Literature: Tetrahedron Letters, , vol. 47, # 15 p. 2549 - 2552 |
|
~% 
19216-56-9 |
| Literature: Tetrahedron Letters, , vol. 47, # 15 p. 2549 - 2552 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |