55395-07-8
| Name | Baohuoside II |
|---|---|
| Synonyms |
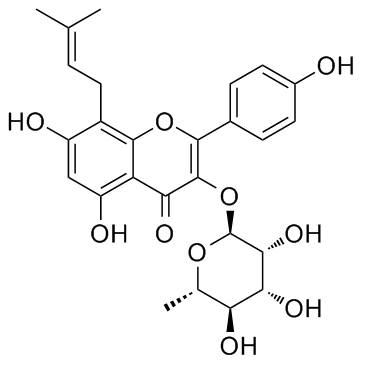
8-((2E)-3-Methylbut-2-enyl)-3-((2S,6S,3R,4R,5R)-3,4,5-trihydroxy-6-methylperhydropyran-2-yloxy)-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
5,7-Dihydroxy-2-(4-hydroxyphenyl)-8-(3-methyl-2-buten-1-yl)-4-oxo-4H-chromen-3-yl 6-deoxy-α-L-mannopyranoside IKarisoside A |
| Description | IKarisoside A(Icarisoside-A) is a natural compound isolated from Epimedium koreanum (Berberidaceae); has anti-inflammatory properties.IC50 value:Target: in vitro: Ikarisoside A inhibited the expression of LPS-stimulated inducible nitric oxide synthase (iNOS) and the production of nitric oxide (NO) in LPS-stimulated RAW 264.7 cells and mouse bone marrow-derived macrophages (BMMs) in a concentration-dependent manner. In addition, Ikarisoside A reduced the release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-1 beta (IL-1 beta). Furthermore, Ikarisoside A inhibited the activity of p38 kinase and nuclear factor-kappaB (NF-kappaB) [1]. Ikarisoside A is a potent inhibitor of osteoclastogenesis in RANKL-stimulated RAW 264.7 cells as well as in bone marrow-derived macrophages.The inhibitory effect of Ikarisoside A resulted in decrease of osteoclast-specific genes like matrix metalloproteinase 9 (MMP9), tartrate-resistant acid phosphatase (TRAP), receptor activator of NF-kappaB (RANK), and cathepsin K. Moreover, Ikarisoside A blocked the resorbing capacity of RAW 264.7 cells on calcium phosphate-coated plates. Ikarisoside A also has inhibitory effects on the RANKL-mediated activation of NF-kappaB, JNK, and Akt [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 782.1±60.0 °C at 760 mmHg |
| Molecular Formula | C26H28O10 |
| Molecular Weight | 500.495 |
| Flash Point | 264.7±26.4 °C |
| Exact Mass | 500.168243 |
| PSA | 170.05000 |
| LogP | 4.47 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.697 |
| Storage condition | 2-8℃ |