38030-57-8
| Name | all-trans-4-oxoretinoic acid |
|---|---|
| Synonyms |
4-keto-Retinoic Acid
4-oxo-all-rans-retinoic acid 4-Oxoretinoic Acid all-trans-4-Oxoretinoic Acid 4-oxo-all-trans-retinoic acid all-trans 4-Keto Retinoic Acid 4-oxo-Retinoic acid 4-Ketoretinoic Acid 4-Oxo-atRA 4-Oxotretinoin |
| Description | all-trans-4-Oxoretinoic acid, an active metabolite of vitamin A, induces gene transcription via binding to nuclear retinoic acid receptors (RARs). |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | all-trans-4-Oxoretinoic acid, an active metabolite of vitamin A, induces gene transcription via binding to nuclear retinoic acid receptors (RARs)[1]. all-trans-4-Oxoretinoic acid is a biologically active geometric isomer of retinoic acid (RA) [2]. |
| References |
| Density | 1.07g/cm3 |
|---|---|
| Boiling Point | 509.9ºC at 760 mmHg |
| Melting Point | 175-178°C (分解) |
| Molecular Formula | C20H26O3 |
| Molecular Weight | 314.41900 |
| Flash Point | 276.3ºC |
| Exact Mass | 314.18800 |
| PSA | 54.37000 |
| LogP | 4.78160 |
| Index of Refraction | 1.565 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
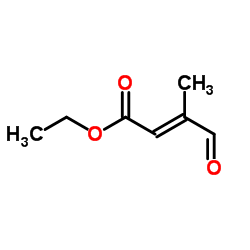
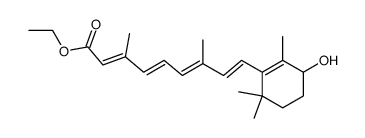
~87% 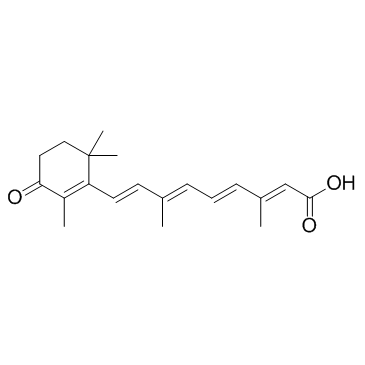
38030-57-8 |
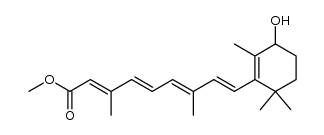
| Literature: Mironova; Leont'eva; Shevyakov; Alexeeva; Shvets; Demina; Krasnokutskaya; Finkel'shtein; Khodonov Russian Journal of Bioorganic Chemistry, 2002 , vol. 28, # 6 p. 487 - 493 |
|
~% 
38030-57-8 |
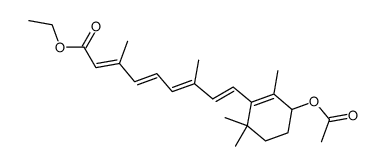
| Literature: Rosenberger Journal of Organic Chemistry, 1982 , vol. 47, # 9 p. 1698 - 1701 |
|
~% 
38030-57-8 |
| Literature: Rosenberger Journal of Organic Chemistry, 1982 , vol. 47, # 9 p. 1698 - 1701 |
|
~% 
38030-57-8 |
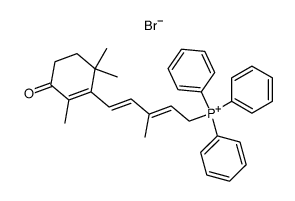
| Literature: Aig, Edward; Focella, Antonino; Parrish, David R.; Rosenberger, Michael; Scott, John W.; Zenchoff, Gladys B. Synthetic Communications, 1987 , vol. 17, # 4 p. 419 - 430 |
|
~% 
38030-57-8 |
| Literature: Aig, Edward; Focella, Antonino; Parrish, David R.; Rosenberger, Michael; Scott, John W.; Zenchoff, Gladys B. Synthetic Communications, 1987 , vol. 17, # 4 p. 419 - 430 |
|
~% 
38030-57-8 |
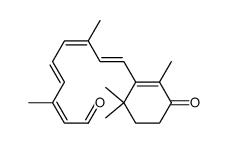
| Literature: UNIVERSITY OF WASHINGTON Patent: US2008/249042 A1, 2008 ; Location in patent: Page/Page column 2; 27; 29; 31; Sheet 14/24; 24/24 ; |
|
~% 
38030-57-8 |
| Literature: Mironova; Leont'eva; Shevyakov; Alexeeva; Shvets; Demina; Krasnokutskaya; Finkel'shtein; Khodonov Russian Journal of Bioorganic Chemistry, 2002 , vol. 28, # 6 p. 487 - 493 |
|
~% 
38030-57-8 |
| Literature: Rosenberger Journal of Organic Chemistry, 1982 , vol. 47, # 9 p. 1698 - 1701 |
|
~% 
38030-57-8 |
| Literature: Aig, Edward; Focella, Antonino; Parrish, David R.; Rosenberger, Michael; Scott, John W.; Zenchoff, Gladys B. Synthetic Communications, 1987 , vol. 17, # 4 p. 419 - 430 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |