10605-03-5
| Name | 2,3,9,10-tetramethoxy-13-methyl-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium,chloride |
|---|---|
| Synonyms |
2,3,9,10-Tetramethoxy-13-methyl-5,6-dihydro-isochino[3,2-a]isochinolinylium,Chlorid
dehydrocorydaline chloride ddehydrocorydaline chloride Dansyl-D-Phe N-[[5-(Dimethylamino)-1-naphthalenyl]sulfonyl]-D-phenylalanine Dansyl-(R)-phenylalanine Dansyl-D-phenylalanine 2,3,9,10-tetramethoxy-13-methyl-5,6-dihydro-isoquino[3,2-a]isoquinolinylium,chloride N-(1-dimethylamino-5-naphthalenesulfonyl)-D-phenylalanine D-Dansylphenylalanine N-Dansyl-D-phenylalanine 13-Methylpalmatine chloride |
| Description | Dehydrocorydaline chloride is an alkaloidal that has anti-inflammatory and anti-cancer activities. Dehydrocorydaline chloride can elevate p38 MAPK activation. |
|---|---|
| Related Catalog | |
| Target |
p38 MAPK[1] |
| In Vitro | Treatment of C2C12 myoblasts with 500 nM Dehydrocorydaline increases the expression levels of muscle-specific proteins, including MyoD, myogenin and myosin heavy chain. Treatment with Dehydrocorydaline elevates p38 MAPK activation and the interaction of MyoD with an E protein. Furthermore, defects in differentiation-induced p38 MAPK activation and myoblast differentiation induced by depletion of the promyogenic receptor protein Cdo in C2C12 myoblasts are restored by Dehydrocorydaline treatment[1]. Dehydrocorydaline significantly inhibits MCF-7 cell proliferation in a dose- dependent manner, which can be reversed by a caspase-8 inhibitor, Z-IETD-FMK. Dehydrocorydaline increases DNA fragments without affecting ΔΨm. Western blotting assay shows that dehydrocorydaline dose-dependently increases Bax protein expression and decreases Bcl-2 protein expression. Furthermore, dehydrocorydaline induces activation of caspase-7,-8 and the cleavage of PARP without affecting caspase-9. These results show that dehydrocorydaline inhibits MCF-7 cell proliferation by inducing apoptosis mediated by regulating Bax/Bcl-2, activating caspases as well as cleaving PARP[3]. |
| In Vivo | Dehydrocorydaline (3.6, 6 or 10 mg/kg, i.p.) shows a dose-dependent antinociceptive effect in the acetic acid-induced writhing test and significantly attenuates the formalin-induced pain responses in mice. In the formalin test, dehydrocorydaline decreases the expression of caspase 6 (CASP6), TNF-α, IL-1β and IL-6 proteins in the spinal cord. These findings confirm that Dehydrocorydaline has antinociceptive effects in mice[2]. |
| Cell Assay | Briefly, MCF-7 cells (1×104 cells/well) are seeded in 96-well plates and treated with different concentrations of dehydrocorydaline (0-200 μM) for 24 h. The cell viability is determined. To explore the role of caspase-8 in dehydrocorydaline induced cytotoxicity, a caspase-8 inhibitor Z-IETD-FMK (10 μM) is co-incubated with 200 μM dehydrocorydaline. |
| Animal Admin | Briefly, the mice are placed individually in glass beakers and are allowed to acclimate for 30 min before the test. The vehicle or Dehydrocorydaline (3.6, 6 or 10 mg/kg) are injected (10 ml/kg, i.p.) 15 min prior to the formalin injection. Morphine (10 mg/kg) or diclofenac sodium (20 mg/kg) are injected 15 and 30 min, respectively, prior to the formalin injection as positive controls. Then, 25 μL of a 5% formalin solution is injected into the plantar surface of the right hind paw of each mouse. Immediately after the formalin injection, the mice are placed individually in the beakers, and a mirror is placed under the beaker to allow clear observation of the paws of the animals. The time that the animals spent on biting/licking the injected paw is measured with a stopwatch every 5 min and considered as indication of nociception. |
| References |
| Density | 1.23g/cm3 |
|---|---|
| Boiling Point | 607.6ºC at 760 mmHg |
| Molecular Formula | C22H24ClNO4 |
| Molecular Weight | 401.88300 |
| Flash Point | 179.9ºC |
| Exact Mass | 401.13900 |
| PSA | 40.80000 |
| LogP | 0.69720 |
| Vapour Pressure | 1.05E-14mmHg at 25°C |
| Index of Refraction | 1.615 |
| Storage condition | -20°C |
|
~84% 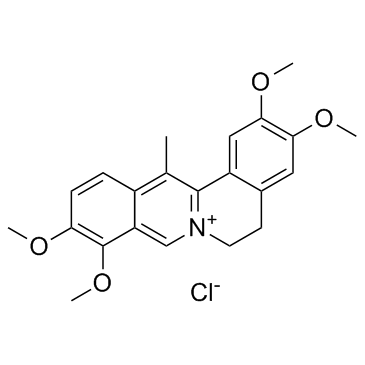
10605-03-5 |
| Literature: Hanaoka; Yoshida; Mukai Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 12 p. 3264 - 3267 |
|
~% 
10605-03-5 |
| Literature: Hanaoka; Yoshida; Mukai Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 12 p. 3264 - 3267 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |