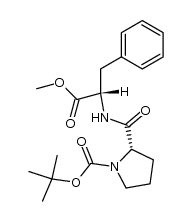
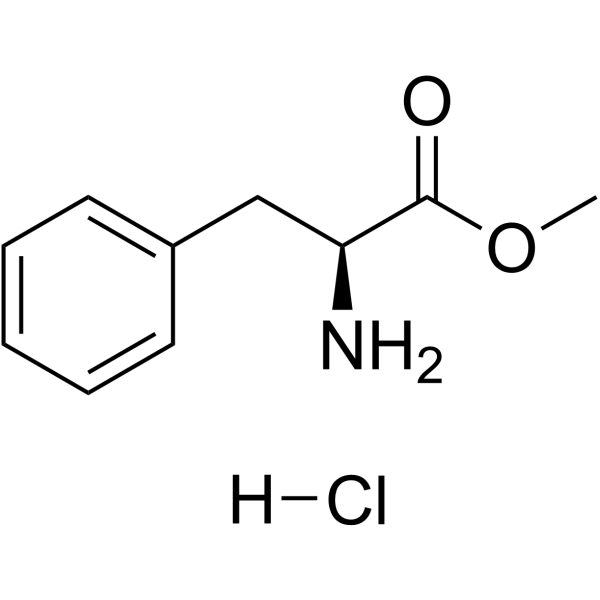
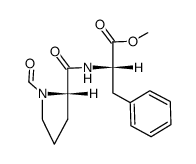
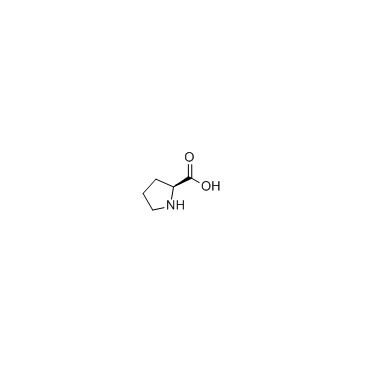
13589-02-1
| Name | h-pro-phe-oh |
|---|---|
| Synonyms |
H-L-Pro-L-Phe-OH
N-L-Prolyl-L-phenylalanine PRO-PHE L-Pro-L-Phe-OH pro-phecrystalline Pro-Phe-OH l-pro-l-phe L-Prolyl-L-phenylalanine PROLINE-PHENYLALANINE |
| Description | H-Pro-Phe-OH is a dipeptide containing proline and phenylalanine, which can serve as a substrate for prolinase. H-Pro-Phe-OH can also be used for polypeptide synthesis, where phenylalanine is an aromatic amino acid that can inhibit the activity of Angiotensin-converting enzyme (ACE, HY-P2983)[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 531.9±50.0 °C at 760 mmHg |
| Molecular Formula | C14H18N2O3 |
| Molecular Weight | 262.30 |
| Flash Point | 275.5±30.1 °C |
| Exact Mass | 262.131744 |
| PSA | 78.43000 |
| LogP | 0.67 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.568 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |