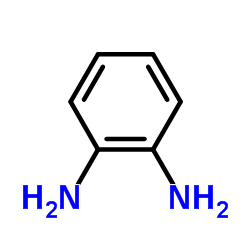
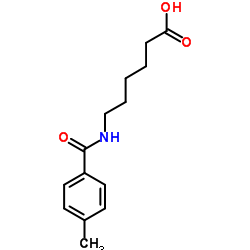
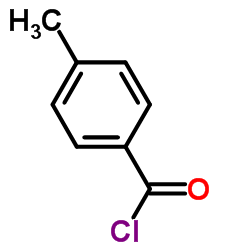
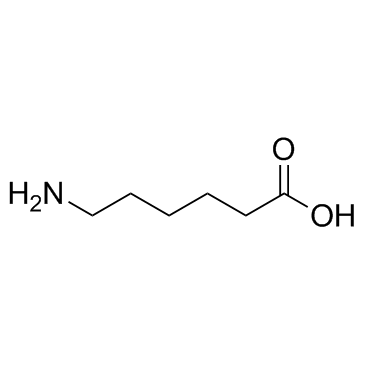
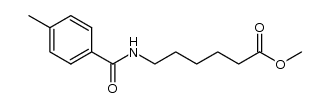
1215493-56-3
| Name | N-[6-(2-aminoanilino)-6-oxohexyl]-4-methylbenzamide |
|---|---|
| Synonyms |
N-(6-(2-aminophenylamino)-6-oxohexyl)-4-methylbenzamide
UNII-17V14R89EU RGFP 109 RG2833 (RGFP109) S7292 RG-2833 RG2833 |
| Description | RG2833 is a brain-penetrant HDAC inhibitor with IC50 of 60 nM and 50 nM for HDAC1 and HDAC3, respectively. |
|---|---|
| Related Catalog | |
| Target |
HDAC3:50 nM (IC50) HDAC1:60 nM (IC50) |
| In Vitro | The Ki values of RG2833 for HDAC1 and HDAC3 are 32 nM and 5 nM, respectively. RG2833 is highly active in the whole tested concentration range from 1 to 10 µM. Continuous incubation with RG2833 slows the increase in frataxin protein, and when the compound is removed, frataxin protein levels rapidly increased in the cells from patient P13[1]. RG2833 produces significant increases in brain aconitase enzyme activity, together with reduction of neuronal pathology of the dorsal root ganglia (DRG)[2]. |
| In Vivo | RG2833 (150 mg/kg) is able to correct frataxin deficiency in the brain and heart of KIKI mice 24 hours after a single injection, but not when lower doses are used. When followed in time, the frataxin mRNA increase induced by RG2833 in the KIKI mouse can be first detected at 12 hours and reach a maximum at 24 hours in both brain and heart[1]. RG2833 (100 mg/kg, s.c.) is well tolerated in chronic dosing of mice without toxicity. RG2833 improves motor coordination of YG8R FRDA mice. RG2833 increases frataxin protein expression in the brain of YG8R FRDA mice[2]. RGFP109 (30 mg/kg, p.o. once daily for 6 days) has no acute effects on dyskinesia after single or 6 days once-daily treatment. One week following cessation of RGFP109, dyskinesia and duration of ON-time with disabling dyskinesia are reduced by 37% and 50%, respectively[3]. |
| Kinase Assay | Aconitase activities are determined by homogenization of mouse brain tissues on ice at 10% w/v in CellLytic MT Mammalian Tissue Lysis/Extraction buffer, followed by centrifugation at 800×g for 10 min at 4°C. Tissue lysates (50 μL) are then added to 200 μL of substrate mix (50 mM Tris/HCl pH 7.4, 0.4 mM NADP, 5 mM Na citrate, 0.6 mM MgCl2, 0.1% (v/v) Triton X-100 and 1U isocitrate dehydrogenase) and the reactions are incubated at 37°C for 15 min, followed by spectrophotometric absorbance measurements every minute for 15 min at 340 nm 37°C to determine the reaction slope. Aconitase activities of mouse brain tissues are then normalized to citrate synthase activities, which are determined using a citrate synthase assay kit. |
| Animal Admin | Mice are housed in conventional open cages with Litaspen Premium 8/20 bedding, paper wool nesting and standard fun tunnel environmental enrichment, with 13 h light, 11 h dark, 20-23°C and 45-60% humidity. The mice are given a diet of SDS RM3 Expanded food pellets and standard drinking water. Mice are given subcutaneous injections of 150 mg/kg RG2833 three times per week for 4.5 months, or 50 mg/kg 136 or 100 mg/kg RG2833 five times per week for 5 months, followed by culling for tissue collection 24 h after the final injection. |
| References |
| Molecular Formula | C20H25N3O2 |
|---|---|
| Molecular Weight | 339.43100 |
| Exact Mass | 339.19500 |
| PSA | 87.71000 |
| LogP | 5.12770 |
| Storage condition | -20℃ |
|
~34% 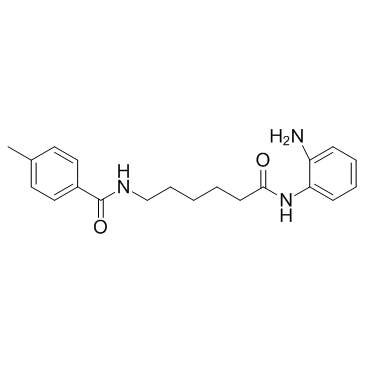
1215493-56-3 |
| Literature: REPLIGEN CORPORATION Patent: US2012/94971 A1, 2012 ; Location in patent: Page/Page column 40 ; |
|
~% 
1215493-56-3 |
| Literature: REPLIGEN CORPORATION Patent: US2012/94971 A1, 2012 ; |
|
~% 
1215493-56-3 |
| Literature: REPLIGEN CORPORATION Patent: US2012/94971 A1, 2012 ; |
|
~% 
1215493-56-3 |
| Literature: REPLIGEN CORPORATION Patent: US2012/94971 A1, 2012 ; |
|
~% 
1215493-56-3 |
| Literature: REPLIGEN CORPORATION Patent: US2012/94971 A1, 2012 ; |
| Precursor 5 | |
|---|---|
| DownStream 0 | |