1186231-83-3
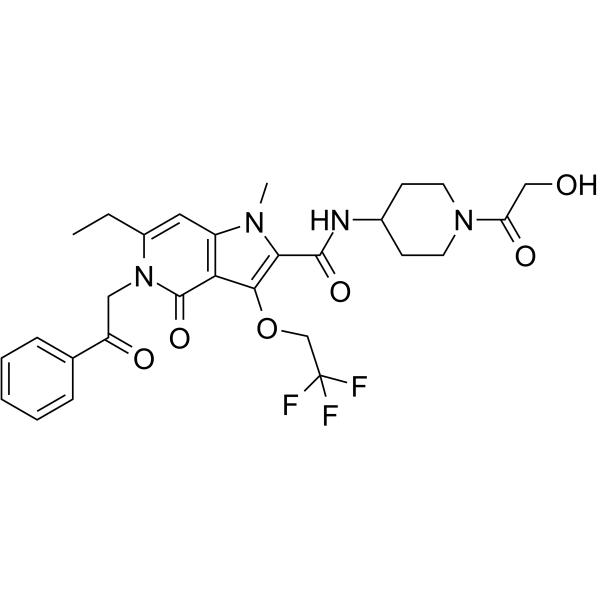
| Name | 6-Ethyl-N-(1-glycoloyl-4-piperidinyl)-1-methyl-4-oxo-5-(2-oxo-2-p henylethyl)-3-(2,2,2-trifluoroethoxy)-4,5-dihydro-1H-pyrrolo[3,2- c]pyridine-2-carboxamide |
|---|---|
| Synonyms |
s-Triazine,4,6-bis(isopropylamino)-2-ethyl
6-ethyl-N-[1-(hydroxyacetyl)piperidin-4-yl]-1-methyl-4-oxo-5-(2-oxo-2-phenylethyl)-3-(2,2,2-trifluoroethoxy)-4,5-dihydro-1H-pyrrolo[3,2-c]pyridine-2-carboxamide 2-Ethyl-4,6-bis(iso-propylamino)-s-triazine 2-ethyl-4,6-bis(isopropylamino)-1,3,5-triazine [11] 6-Ethyl-N-[1-(hydroxyacetyl)piperidin-4-yl]-1-methyl-4-oxo-5-(2-oxo-2-phenylethyl)-3-(2,2,2-trifluoroethoxy)-4,5-dihydro-1H-pyrrolo[3,2-c]pyridine-2-carboxamide 6-ethyl-N,N'-diisopropyl-[1,3,5]triazine-2,4-diamine 4,6-Bis(isopropylamino)-2-ethyl-s-triazine 6-Ethyl-N-(1-glycoloyl-4-piperidinyl)-1-methyl-4-oxo-5-(2-oxo-2-phenylethyl)-3-(2,2,2-trifluoroethoxy)-4,5-dihydro-1H-pyrrolo[3,2-c]pyridine-2-carboxamide 6-ethyl-N,N'-di(propan-2-yl)-1,3,5-triazine-2,4-diamine |
| Description | TAK-441 (compound 11d) is a highly potent and oral hedgehog (Hh) signaling inhibitor with an IC50value of 4.4 nM. TAK-441 (compound 11d) has strong antitumor activity in solid tumors[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 4.4 nM (Gli-luc reporter)[1] |
| In Vitro | TAK-441 (compound 11d) has potent activity in the Gli-luc reporter with an IC50 value of 4.4 nM and good solubility. TAK-441 inhibits Gli1 mRNA with IC50 values of 0.0457 and 0.113 mg/ml in the tumor and skin, respectively. TAK-441 does not affect androgen withdrawal-induced Shh up-regulation orviability of LNCaP cells. TAK-441 leads to delayed castration-resistant progression of LNCaP xenografts by disrupting paracrine Hh signaling with the tumor stroma[1][2][3]. Cell Viability Assay[1] Cell Line: NIH3T3/Gli-luc cells Concentration: 0.03–1000 nM Incubation Time: 48 h Result: Showed acceptable solubility and potent Hh inhibitory activity. Cell Viability Assay[3] Cell Line: LNCaP cells Concentration: 0.5-500 nM Incubation Time: 48-72 h Result: Did not affect up-regulation of Shh of in vitro viability of LNCaP cells under androgen-deprivedconditionsin. Western Blot Analysis[3] Cell Line: LNCaP, C4-2, DU145 and PC3 cells Concentration: Incubation Time: Result: Reflected androgen-responsive PCa and express both Shh and Dhh in LNCaP and C4-2 cells and reflect restricted Shh expression of CRPC in DU145 and PC3 cells. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 761.6±60.0 °C at 760 mmHg |
| Molecular Formula | C28H31F3N4O6 |
| Molecular Weight | 576.564 |
| Flash Point | 414.4±32.9 °C |
| Exact Mass | 576.219543 |
| PSA | 126.36000 |
| LogP | 2.64 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.606 |
|
~% 
1186231-83-3 |
| Literature: Takeda Pharmaceutical Company Limited Patent: EP2471791 A1, 2012 ; |
|
~% 
1186231-83-3 |
| Literature: Takeda Pharmaceutical Company Limited Patent: EP2471791 A1, 2012 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |