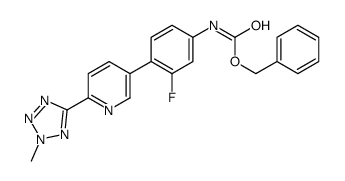
856866-72-3
| Name | tedizolid |
|---|---|
| Synonyms |
(5R)-3-{3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl}-5-(hydroxymethyl)-1,3-oxazolidin-2-one
Torezolid TR-701 (5R)-3-[3-fluoro-4-[6-(2-methyltetrazol-5-yl)pyridin-3-yl]phenyl]-5-(hydroxymethyl)-1,3-oxazolidin-2-one TR-700 (R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-one Tedizolid [USAN:INN] UNII:97HLQ82NGL Tedizolid (5R)-3-{3-fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl]phenyl}-5-(hydroxymethyl)-2-oxazolidinone |
| Description | Tedizolid is a novel oxazolidinone, acting through inhibition of bacterial protein synthesis by binding to 23S ribosomal RNA (rRNA) of the 50S subunit of the ribosome. |
|---|---|
| Related Catalog | |
| In Vitro | Tedizolid (0.25 μg/mL) inhibits all 28 clinical isolates of PRSP, and is 4-fold more potent than linezolid against PRSP[1]. |
| In Vivo | For mice infected with PSSP type III, the 100% survival rate is achieved with tedizolid phosphate at a minimum total daily dose of 10 mg/kg. Lungs of infected mice treated with tedizolid phosphate show less severe inflammation and edema, as indicated by the mean scores for inflammation and edema[1]. |
| Animal Admin | To induce a systemic S. pneumoniae infection, male ICR mice (weight, 18 to 20 g) are inoculated intraperitoneally with 1 of 4 PRSP isolates (DR9, DR10, DR11, or DR14) suspended in 10% mucin. The suspension contained sufficient bacteria to kill 100% of untreated control mice. At 1 h postinfection, mice receives a single dose of either tedizolid phosphate or linezolid, and survival is assessed daily for 7 days postinfection. Treatments are delivered both orally and intravenously at each of four doses (40 mg/kg of body weight, 13.33 mg/kg, 4.44 mg/kg, and 1.48 mg/kg), with 8 mice per group defined by dose, delivery method, and infecting strain. The 50% effective dose (ED50), i.e., the dose allowing survival of 50% of the infected mice, is calculated for each delivery route using probit analysis. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 614.5±65.0 °C at 760 mmHg |
| Molecular Formula | C17H15FN6O3 |
| Molecular Weight | 370.338 |
| Flash Point | 325.4±34.3 °C |
| Exact Mass | 370.118958 |
| PSA | 106.26000 |
| LogP | 1.56 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.725 |
| HS Code | 29339900 |
|---|
|
~84% 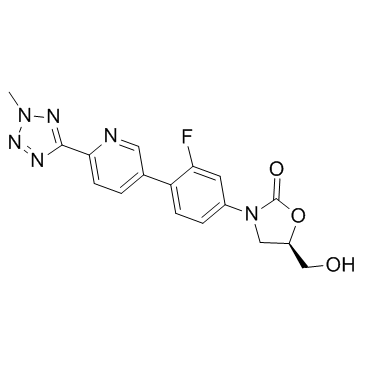
856866-72-3 |
| Literature: TRIUS THERAPEUTICS; COSTELLO, Carrie, A.; SIMSON, Jaqueline, A.; DUGUID, Robert, J.; PHILLIPSON, Douglas Patent: WO2010/42887 A2, 2010 ; Location in patent: Page/Page column 19-20 ; |
|
~% 
856866-72-3 |
| Literature: European Journal of Medicinal Chemistry, , vol. 46, # 4 p. 1027 - 1039 |
|
~% 
856866-72-3 |
| Literature: European Journal of Medicinal Chemistry, , vol. 46, # 4 p. 1027 - 1039 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |