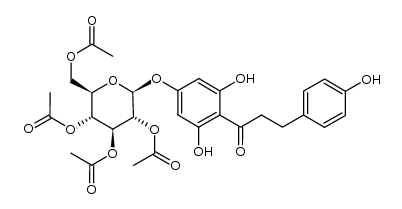
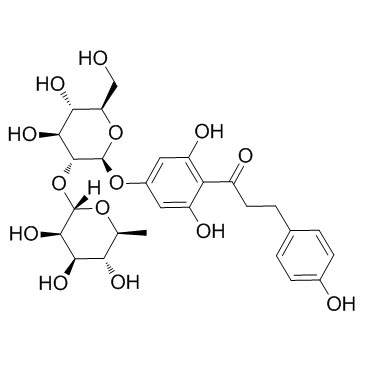
4192-90-9
| Name | 1-[2,6-dihydroxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-3-(4-hydroxyphenyl)propan-1-one |
|---|---|
| Synonyms |
UNII:23298I791N
3,5-Dihydroxy-4-[3-(4-hydroxyphenyl)propanoyl]phenyl β-D-glucopyranoside p-Phloridzin phloretin-4-D-glucoside T6OTJ BOR CQ EQ DV2R DQ&& CQ DQ EQ F1Q &&β-D-Gluco Form p-Phlorizin Trilobatin 1-(4-(β-D-Glucopyranosyloxy)-2,6-dihydroxyphenyl)-3-(4-hydroxyphenyl)-1-propanone |
| Description | Trilobatin, a natural sweetener derived from Lithocarpus polystachyus Rehd[1], Trilobatin is an HIV-1 entry inhibitor targeting the HIV-1 Gp41 envelope[2]. Neuroprotective effects[1]. Trilobatin is also a SGLT1/2 inhibitor that selectively induces the proliferation of human hepatoblastoma cells[3]. |
|---|---|
| Related Catalog | |
| Target |
HIV-1 SGLT1/2 |
| In Vitro | Trilobatin protects against oxidative injury in neuronal PC12 cells through regulating mitochondrial reactive oxygen species (mtROS) homeostasis in the first time, which is, at least partly, mediated through the AMPK/Nrf2/Sirt3 signaling pathway[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 787.9±60.0 °C at 760 mmHg |
| Melting Point | 163 °C |
| Molecular Formula | C21H24O10 |
| Molecular Weight | 436.409 |
| Flash Point | 277.1±26.4 °C |
| Exact Mass | 436.136932 |
| PSA | 177.14000 |
| LogP | 1.43 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.686 |
|
~49% 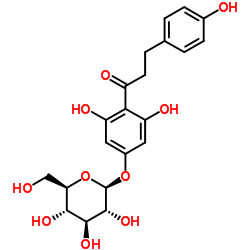
4192-90-9 |
| Literature: Gupte, Amol; Buolamwini, John K. Bioorganic and Medicinal Chemistry Letters, 2009 , vol. 19, # 3 p. 917 - 921 |
|
~% 
4192-90-9 |
| Literature: Jorio Annali di Chimica (Rome, Italy), 1959 , vol. 49, p. 1929,1936 |
|
~% 
4192-90-9 |
| Literature: Jorio Annali di Chimica (Rome, Italy), 1959 , vol. 49, p. 1929,1936 |
|
~% 
4192-90-9 |
| Literature: Jorio Annali di Chimica (Rome, Italy), 1959 , vol. 49, p. 1929,1936 |
| Precursor 4 | |
|---|---|
| DownStream 1 | |