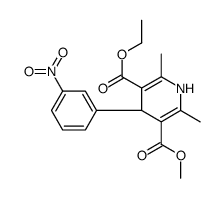
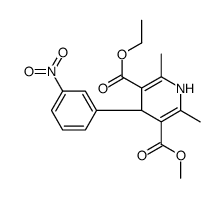
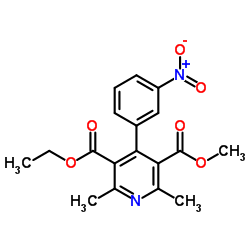
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
US5653000
-
CHEMICAL NAME :
-
3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, ethyl methyl ester
-
CAS REGISTRY NUMBER :
-
39562-70-4
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C18-H20-N2-O6
-
MOLECULAR WEIGHT :
-
360.40
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
15370 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes Blood - changes in spleen
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,121,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
205 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes Blood - changes in spleen
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,121,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5166 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Blood - changes in spleen Skin and Appendages - hair
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,121,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
12600 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
52EDA6 "Nitrendipine, Based on Presentations at International Workshop on Nitrendipine, 1st, New York, 1982," Scriabine, A., et al., eds., Baltimore, Urban & Schwarzenberg, 1984 Volume(issue)/page/year: -,25,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2540 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - rigidity (including catalepsy) Lungs, Thorax, or Respiration - cyanosis
-
REFERENCE :
-
52EDA6 "Nitrendipine, Based on Presentations at International Workshop on Nitrendipine, 1st, New York, 1982," Scriabine, A., et al., eds., Baltimore, Urban & Schwarzenberg, 1984 Volume(issue)/page/year: -,25,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
303 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - other changes Blood - changes in spleen
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,121,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
7613 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Blood - changes in spleen Skin and Appendages - hair
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,121,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
34500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FRPSAX Farmaco, Edizione Scientifica. (Casella Postale 227, 27100 Pavia, Italy) V.8-43 1953-88 For publisher information, see FRMCE8 Volume(issue)/page/year: 42,697,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>100 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
52EDA6 "Nitrendipine, Based on Presentations at International Workshop on Nitrendipine, 1st, New York, 1982," Scriabine, A., et al., eds., Baltimore, Urban & Schwarzenberg, 1984 Volume(issue)/page/year: -,25,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>2500 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
52EDA6 "Nitrendipine, Based on Presentations at International Workshop on Nitrendipine, 1st, New York, 1982," Scriabine, A., et al., eds., Baltimore, Urban & Schwarzenberg, 1984 Volume(issue)/page/year: -,25,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - rigidity (including catalepsy) Lungs, Thorax, or Respiration - cyanosis
-
REFERENCE :
-
52EDA6 "Nitrendipine, Based on Presentations at International Workshop on Nitrendipine, 1st, New York, 1982," Scriabine, A., et al., eds., Baltimore, Urban & Schwarzenberg, 1984 Volume(issue)/page/year: -,25,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2500 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
52EDA6 "Nitrendipine, Based on Presentations at International Workshop on Nitrendipine, 1st, New York, 1982," Scriabine, A., et al., eds., Baltimore, Urban & Schwarzenberg, 1984 Volume(issue)/page/year: -,25,1984 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
107 gm/kg/2Y-C
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
52EDA6 "Nitrendipine, Based on Presentations at International Workshop on Nitrendipine, 1st, New York, 1982," Scriabine, A., et al., eds., Baltimore, Urban & Schwarzenberg, 1984 Volume(issue)/page/year: -,25,1984 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1720 mg/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - behavioral Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,133,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
11000 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,145,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1100 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,145,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
330 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 36,145,1988
|






![methyl 2-methyl-4-(3-nitrophenyl)-5-oxo-7H-furo[3,4-b]pyridine-3-carboxylate structure](https://image.chemsrc.com/caspic/133/64603-74-3.png)