946846-83-9
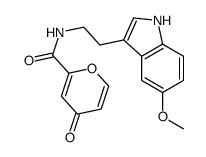
| Name | N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-4-oxopyran-2-carboxamide |
|---|---|
| Synonyms |
NEU-P11
UNII-S3UN2146K9 Piromelatine |
| Description | Piromelatine (Neu-P11) is a melatonin MT1/MT2 receptor agonist, serotonin 5-HT1A/5-HT1D agonist, and serotonin 5-HT2B antagonist. Piromelatine (Neu-P11) possesses sleep promoting, analgesic, anti-neurodegenerative, anxiolytic and antidepressant potentials. Piromelatine (Neu-P11) also possesses pain-related P2X3, TRPV1, and Nav1.7 channel-inhibition capacities[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
MT1 MT2 5-HT1A Receptor 5-HT1D Receptor 5-HT2B Receptor |
| In Vivo | Piromelatine (20 mg/kg, ip, daily) treatment prevents insulin resistance induced by sleep restriction[1]. Piromelatine (5-50 mg/kg, ip, daily) decreases plasma glucose significantly[2]. Piromelatine (100 mg/kg) decreases thermal hyperalgesia and mechanical allodynia in PSL (partial sciatic nerve ligation) mice[3]. Animal Model: Twenty four male Sprague-Dawley rats (3 months old, weighing 250-300 g)[1]. Dosage: 20 mg/kg. Administration: IP, daily at 8:00 p.m. Result: Resulted in significantly decreased plasma glucose levels (6.670.35 mmol/L, 6.770.34 mmol/L vs. 8.27 0.38 mmol/L), and the plasma glucose levels of the two groups were even neared to that of the normal control group (6.07±0.35 mmol/L). Resulted in a decrease in triglycerides and total cholesterol levels (51.8% and43.0%, respectively) and an elevation in HDL-C level (increase of 32.4%). Animal Model: Five groups of 12-wk-old rats (10/group)[2]. Dosage: 5-50 mg/kg. Administration: Intraperitoneal injection in 18:00 every day. Result: Plasma glucose was decreased significantly by 27.3%, 34.5% and 61.5%, respectively. Animal Model: Male C57BL/6 J mice, weighing 22-26 g (10 weeks old; PSL mice)[3]. Dosage: 25, 50, or 100 mg/kg. Administration: IP 1 h before assessment of thermal hyperalgesia and mechanical allodynia. Result: Remarkably prolonged thermal latency (surgery×treatment interaction, F1,24=15.7, p<0.001; surgery×treatment×hours interaction, F5,120=3.0, p<0.05) and increased mechanical threshold (surgery×treatment interaction, F1,24=18.4, p<0.001; surgery× treatment×hours interaction, F5,120=2.6, p<0.05) for 4 h after administration of piromelatine to PSL mice. |
| References |
| Molecular Formula | C17H16N2O4 |
|---|---|
| Molecular Weight | 312.32000 |
| Exact Mass | 312.11100 |
| PSA | 84.33000 |
| LogP | 2.49310 |