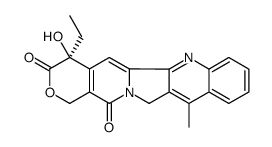
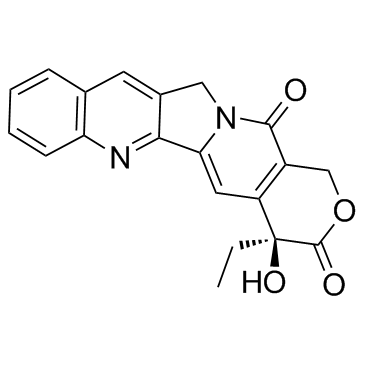
213819-48-8
| Name | Belotecan hydrochloride |
|---|---|
| Synonyms |
Belotecan HCl
Camtobell hydrochloride CKD 602 Belotecan (hydrochloride) |
| Description | Belotecan hydrochloride (CKD-602 hydrochloride), a Topoisomerase I inhibitor, is a synthetic and water-soluble camptothecin derivative. |
|---|---|
| Related Catalog | |
| Target |
Top1 |
| In Vitro | Belotecan exerts a significant cytotoxic effect on YD-8, YD-9 and YD-38 cells in a time- and dose-dependent manner with IC50 values of 2.4, 0.18 and 0.05 μg/mL at 72 h following treatment. Belotecan induces apoptosis in these cell lines. Belotecan induces G2/M phase arrest in oral squamous cell cancer cells[1]. Belotecan shows a significant anticancer effect on glioma cells, with IC50 values of 9.07 nM for LN229, 14.57 nM for U251 MG, 29.13 nM for U343 MG, and 84.66 nM for U87 MG[2]. |
| In Vivo | Belotecan has a significant effect on intracerebral glioma growth, with animals having significantly smaller tumors than those in the control group[3]. |
| Cell Assay | The cells are treated with different concentrations (0.01, 0.1, 0.5, 1, 5 and 10 μg/mL) of belotecan for 24, 48 and 72 h. Control samples of each cell line are treated with medium only. Cell viability is measured using the MTS assay[1]. |
| Animal Admin | Mice: Nude mice with established U87MG glioma are treated with a dose of belotecan of 0 mg/kg (control group, injection with saline), 40 mg/kg (group A) or 60 mg/kg (group B). Thereafter, the dose is repeated once every 4 days for a total of four doses. Tumor volume is measured histologically and apoptosis is detected[1]. |
| References |
| Boiling Point | 772.4ºC at 760mmHg |
|---|---|
| Molecular Formula | C25H28ClN3O4 |
| Molecular Weight | 469.96100 |
| Flash Point | 420.9ºC |
| Exact Mass | 469.17700 |
| PSA | 93.45000 |
| LogP | 3.81300 |
| Vapour Pressure | 4.21E-25mmHg at 25°C |
| Storage condition | 2-8℃ |
|
~46% 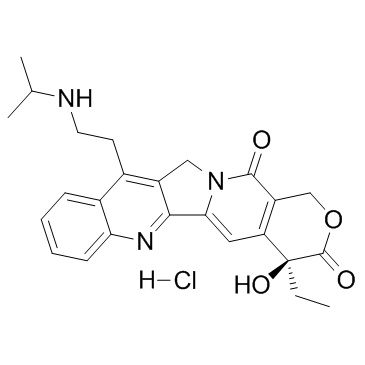
213819-48-8 |
| Literature: Ahn, Soon Kil; Choi, Nam Song; Jeong, Byeong Seon; Kim, Kye Kwang; Journ, Duck Jin; Kim, Joon Kyum; Lee, Sang Joon; Kim, Jung Woo; Hong, Chung Il; Jew, Sang-sup Journal of Heterocyclic Chemistry, 2000 , vol. 37, # 5 p. 1141 - 1144 |
|
~% 
213819-48-8 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 37, # 5 p. 1141 - 1144 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |