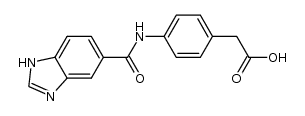
99764-63-3
| Name | h-leu-ser-p-nitro-phe-nle-ala-leu-ome tfa |
|---|---|
| Synonyms |
LEU-SER-P-NITRO-PHE-NLE-ALA-LEU-METHYL ESTER TFA
LEU-SER-PHE(NO2)-NLE-ALA-LEU-OME H-Leu-Ser-p-nitro-Phe-Nle-Ala-Leu-OMe H-LEU-SER-PHE(4-NO2)-NLE-ALA-LEU-OME TFA Leu-Ser-p-nitro-Phe-Nle-Ala-Leu methyl ester LEUCINE-SERINE-P-NITRO-PHENYLALANINE-NLE-ALANINE-LEUCINE METHYL ESTER LSF(NO2)-NLE-AL-METHYL ESTER |
| Description | H-Leu-Ser-Phe(NO2)-Nle-Ala-OMe TFA is a substrate for chymosin[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C36H56F3N7O12 |
|---|---|
| Molecular Weight | 835.86500 |
| Exact Mass | 835.39400 |
| PSA | 301.17000 |
| LogP | 4.16750 |
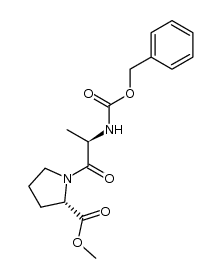
|
~88% 
99764-63-3 |
| Literature: Urbanski, Maud J.; Gunnet JR., Joseph W.; Demarest, Keith T. Patent: US2003/73842 A1, 2003 ; US 20030073842 A1 |
|
~% 
99764-63-3 |
| Literature: Aventis Pharmaceuticals Inc. Patent: US6541505 B1, 2003 ; US 6541505 B1 |
|
~% 
99764-63-3 |
| Literature: Kanebo, Ltd Patent: US5919769 A1, 1999 ; |
|
~%
Detail
|
| Literature: Merck and Co., Inc. Patent: US5464788 A1, 1995 ; |
|
~%
Detail
|
| Literature: Merck and Co., Inc. Patent: US5464788 A1, 1995 ; |
|
~% 
99764-63-3 |
| Literature: Bristol-Myers Squibb Company Patent: US5583146 A1, 1996 ; US 5583146 A |
| Precursor 10 | |
|---|---|
| DownStream 0 | |