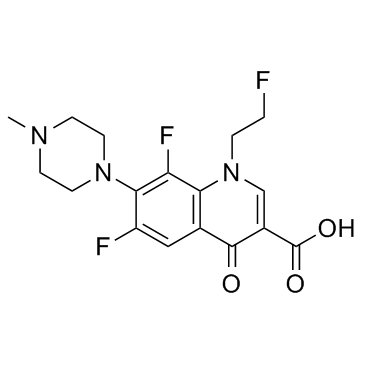
79660-72-3
| Name | Fleroxacin |
|---|---|
| Synonyms |
Fleroxacin
6,8-difluoro-1-(2-fluoroethyl)-1,4-dihydro-4-oxo-7-(4-methyl-1-piperazinyl)-quinoline-3-carboxylic acid MEGALONE 6,8-difluoro-1-(2-fluoro-ethyl)-1,4-dihydro-7-(4-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid 6,8-Difluoro-1-(2-fluoroethyl)-7-(4-methyl-1-piperazinyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid ro23-6240/000 Megalocin AM-833 Quinodis 3-quinolinecarboxylicacid,1,4-dihydro-6,8-difluoro-1-(2-fluoroethyl)-7-(4-met hyl-1-piperazinyl)-4-oxo MFCD00864880 6,8-difluoro-1-(2-fluoroethyl)-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid 6,8-Difluoro-1-(2-fluoroethyl)-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 6,8-difluoro-1-(2-fluoroethyl)-1,4-dihydro-7-(4-methyl-1-piperazinyl)-4-oxoquinoline-3-carboxylic acid RO-23-6240 |
| Description | Fleroxacin is a broad-spectrum antimicrobial fluoroquinolone.Target: AntibacterialFleroxacin (Ro 23-6240; AM-833) is a new trifluorinated quinolone exhibiting high activity against a broad spectrum of gram-negative and gram-positive bacteria. Fleroxacin is characterized pharmacokinetically by a long elimination half-life (9 to 10 h) and high concentrations in plasma (e.g., maximum concentration of 2.3 micrograms/ml after an oral dose of 200 mg) [1]. Fleroxacin is effective against Haemophilus ducreyi in vitro. Fleroxacin, 200 or 400 mg as a single oral dose, is efficacious therapy for microbiologically proven chancroid in patients who do not have concurrent HIV-1 infection. Among HIV-1-infected men, a single dose of 200 or 400 mg of fleroxacin is inadequate therapy for chancroid [2, 3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 535.3±50.0 °C at 760 mmHg |
| Melting Point | 264-266°C |
| Molecular Formula | C17H18F3N3O3 |
| Molecular Weight | 369.338 |
| Flash Point | 277.6±30.1 °C |
| Exact Mass | 369.130035 |
| PSA | 65.78000 |
| LogP | 1.72 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.568 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| RIDADR | NONH for all modes of transport |
| RTECS | VB1999050 |
| HS Code | 2933990090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |