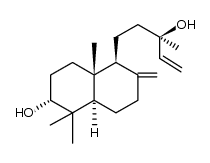
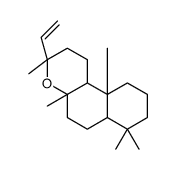
596-85-0
| Name | manool |
|---|---|
| Synonyms |
8,14-labdadien-13-ol
MFCD01310996 MANOOL |
| Description | Manool is a diterpene from Salvia officinalis. Manool induces selective cytotoxicity in cancer cells. Manool arrests the cancer cells at the G(2)/M phase of the cell cycle[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Manool exhibits higher cytotoxic activity against HeLa (IC50=6.7 µg/mL) and U343 (IC50=6.7 µg/mL) cells[1]. Manool exhibits a protective effect against chromosome damage induced by MMS in HepG2 cells[3]. |
| References |
[2]. Pratsinis H, et al. Antiproliferative activity of Greek propolis. J Med Food. 2010;13(2):286-290. |
| Density | 0.93g/cm3 |
|---|---|
| Boiling Point | 368.2ºC at 760mmHg |
| Melting Point | 49-52ºC(lit.) |
| Molecular Formula | C20H34O |
| Molecular Weight | 290.48300 |
| Flash Point | 118.2ºC |
| Exact Mass | 290.26100 |
| PSA | 20.23000 |
| LogP | 5.50240 |
| Index of Refraction | 1.5 |
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| WGK Germany | 3 |
|
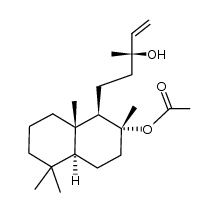
~91% 
596-85-0 |
| Literature: Rogachev, Victor; Loehl, Thorsten; Markert, Thomas; Metz, Peter Arkivoc, 2012 , vol. 2012, # 3 p. 172 - 180 |
|
~15% 
596-85-0 
596-85-0 |
| Literature: Organic and Biomolecular Chemistry, , vol. 9, # 7 p. 2156 - 2165 |
|
~% 
596-85-0 |
| Literature: Arkivoc, , vol. 2012, # 3 p. 172 - 180 |
|
~% 
596-85-0 |
| Literature: Arkivoc, , vol. 2012, # 3 p. 172 - 180 |
|
~% 
596-85-0 |
| Literature: Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, , vol. 26, # 1-12 p. 453 - 458 |
|
~% 
596-85-0 |
| Literature: Agricultural and Biological Chemistry, , vol. 46, # 10 p. 2477 - 2484 |
|
~%
Detail
|
| Literature: Justus Liebigs Annalen der Chemie, , vol. 617, p. 134 Journal of the Chemical Society, , p. 2187,2189 |
| Precursor 7 | |
|---|---|
| DownStream 6 | |