692-13-7
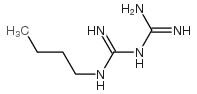
| Name | 1-butylbiguanide |
|---|---|
| Synonyms |
Glybigid
Buformine W 37 Dibetos B Butformin 1-butyl-biguanide Butylbiguanide N-butylbiguanide 1-Butyl-biguanid DBV H 224 Buformin Butyldiguanide EINECS 211-726-4 |
| Description | Buformin (1-Butylbiguanide) is a potent and orally active biguanide antidiabetic agent, an AMPK activator. Buformin decreases hepatic gluconeogenesis and lowers blood glucose production in vivo. Buformin also has anti-cancer activities and is applied in cancer study (such as, cervical cancer and breast cancer, et al)[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Buformin (0-10 mM; 5 days) inhibits SKBR3 and BT474 cells growth as a concentration-dependent manner, exhibits IC50 values of 246.7 μM and 98.6 μM for erbB-2-overexpressing SKBR3 and BT474 cells, respectively[1]. Buformin (0-3 mM; 48 hours) increases the percentage of cells in G0/G1 phase and reduced the percentage of cells in S phase, especially in the SKBR3 cells[1]. Buformin (0-3 mM; 24 hours) suppresses RTK activation, including erbB-2 and IGF1R signaling downstream, and Akt activation/phosphorylation is inhibited in both SKBR3 and BT474 cells[1]. Cell Viability Assay[1] Cell Line: ErbB-2-overexpressing SKBR3 and BT474 cells Concentration: 0 μM, 1 μM, 3 μM, 10 μM, 30 μM, 100 μM, 300 μM, 1, 3, or 10 mM Incubation Time: 5 days Result: Reduced cell viability in erbB-2-overexpressing breast cells. Cell Cycle Analysis[1] Cell Line: ErbB-2-overexpressing SKBR3 and BT474 cells Concentration: 0.5 mM; 1 mM; 3 mM Incubation Time: 48 hours Result: Increased cells arresting in G0/G1 phase. Western Blot Analysis[1] Cell Line: ErbB-2-overexpressing SKBR3 and BT474 cells Concentration: 0 mM, 0.1 mM, 0.3 mM, 1 mM, or 3 mM Incubation Time: 24 hours Result: Decreased p-AMPK, p-p706S, p-ERK1/2 expression in a concentration-dependent manner. |
| In Vivo | Buformin (oral administation; 7.6 mmol/kg of chow; 7 days) exhibits significantly reduced tumor volumes and weights, and hinders mammary morphogenesis and proliferation in MMTV-erbB-2 transgenic mice[1] Animal Model: Female MMTV-erbB-2 transgenic mice[1] Dosage: 7.6 mmol/kg Administration: Oral administation; 7 days Result: Inhibited mammary syngeneic tumor growth in MMTV-erbB-2 transgenic mice. |
| References |
| Density | 1.22g/cm3 |
|---|---|
| Boiling Point | 322.7ºC at 760 mmHg |
| Molecular Formula | C6H15N5 |
| Molecular Weight | 157.21700 |
| Flash Point | 148.9ºC |
| Exact Mass | 157.13300 |
| PSA | 97.78000 |
| LogP | 1.47550 |
| Index of Refraction | 1.568 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
692-13-7 |
| Literature: Dutta,R.L.; Sengupta,N.R. Journal of the Indian Chemical Society, 1961 , vol. 38, p. 741 - 746 |
|
~% 
692-13-7 |
| Literature: Shaw; Gross Journal of Organic Chemistry, 1959 , vol. 24, p. 1809 Full Text View citing articles Show Details Shapiro et al. Journal of the American Chemical Society, 1959 , vol. 81, p. 3725,3726 |
|
~% 
692-13-7 |
| Literature: Urbanski et al. Roczniki Chemii, 1959 , vol. 33, p. 1383,1387 Chem.Abstr., 1960 , p. 13034 |