13312-52-2
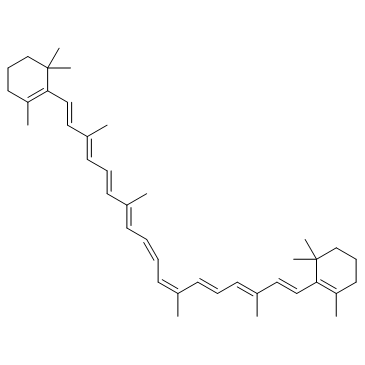
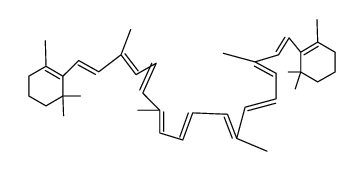
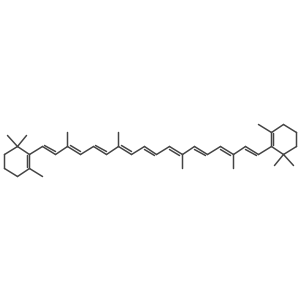
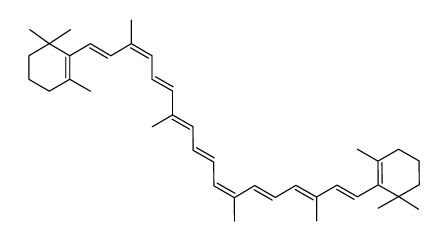
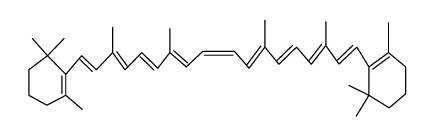
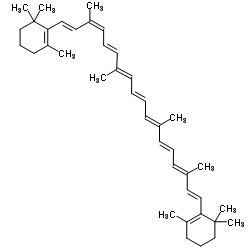
| Name | 1,3,3-trimethyl-2-[(1E,3Z,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohexene |
|---|---|
| Synonyms |
neo U-|A-Carotene
9-cis-|A,|A-Carotene 9-CIS-BETA-CAROTENE (9cis)-β,β-Carotene Neo-|A-carotene U 9-cis-|A-Carotene |
| Description | 9-cis-β-Carotene, a precursor of retinal, is cleaved by beta-carotene oxygenase 1 (BCMO1) to produce 9-cis-retinal. 9-cis-β-Carotene inhibits photoreceptor degeneration and restores retinal function in vivo. 9-cis-β-Carotene has the potential for the study of congenital stationary night blindness and fundus albipunctatus[1]. |
|---|---|
| Related Catalog | |
| In Vitro | 9-cis-β-Carotene (1 μM; 18 hours) formulates with the Miglyol 810 and the“antioxidant mix” is incubated to eye cups. The eye cups are isolated from the neuro-retina attached to the underlying RPE tissue from RPE65rd12 mouse. It prevents S- and M-cones degeneration and leads to over 2- and 9- fold higher m-opsin and s-opsin positive staining compares to the control group[1]. |
| In Vivo | In a mouse model of retinoid cycle defect, RPE65rd12. Mice with an extract of Dunaliella Bardawil algae rich in 9-cis-β-Carotene (9CBC) rescues retinal function and preserves cone structure by oral administration[1]. |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 654.7±22.0 °C at 760 mmHg |
| Molecular Formula | C40H56 |
| Molecular Weight | 536.873 |
| Flash Point | 346.0±17.2 °C |
| Exact Mass | 536.438232 |
| LogP | 15.51 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.566 |
|
~% 
13312-52-2 |
| Literature: Journal of the American Chemical Society, , vol. 64, p. 1856,1859 Journal of the American Chemical Society, , vol. 66, p. 186,190 |
|
~% 
13312-52-2 |
| Literature: Journal of the American Chemical Society, , vol. 104, # 22 p. 6117 - 6119 |
|
~% 
13312-52-2 |
| Literature: Industrial and Engineering Chemistry, Analytical Edition, , vol. 16, p. 328 Archives of Biochemistry, , vol. 5, p. 211 Journal of the American Chemical Society, , vol. 64, p. 1856,1859 Journal of the American Chemical Society, , vol. 66, p. 186,190 Journal of the American Chemical Society, , vol. 66, p. 137,142, 143 |
|
~% 
13312-52-2 |
| Literature: Industrial and Engineering Chemistry, Analytical Edition, , vol. 16, p. 328 Archives of Biochemistry, , vol. 5, p. 211 Journal of the American Chemical Society, , vol. 64, p. 1856,1859 Journal of the American Chemical Society, , vol. 66, p. 186,190 Journal of the American Chemical Society, , vol. 66, p. 137,142, 143 |
|
~% 
13312-52-2 |
| Literature: Industrial and Engineering Chemistry, Analytical Edition, , vol. 16, p. 328 Archives of Biochemistry, , vol. 5, p. 211 Journal of the American Chemical Society, , vol. 64, p. 1856,1859 Journal of the American Chemical Society, , vol. 66, p. 186,190 Journal of the American Chemical Society, , vol. 66, p. 137,142, 143 |
|
~% 
13312-52-2 |
| Literature: JAOCS, Journal of the American Oil Chemists' Society, , vol. 75, # 7 p. 823 - 829 |
|
~% 
13312-52-2 |
| Literature: Journal of Physical Chemistry, , vol. 95, # 19 p. 7171 - 7180 |
|
~% 
13312-52-2
Detail
|
| Literature: Journal of Physical Chemistry, , vol. 95, # 19 p. 7171 - 7180 |
| Precursor 5 | |
|---|---|
| DownStream 3 | |