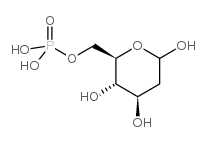
1949-89-9
| Name | 2-deoxy-D-galactose |
|---|---|
| Synonyms |
2-DEOXY-D-LYXOHEXOSE
2-deoxy-galactose 2-deoxy-lyxo-hexose 2-Deoxy-B-D-Lyxo-Hexose EINECS 217-765-3 Deoxygalactose 2-Dexoy-D-Lyxohexose D-2-Desoxygalactose D-2-deoxygalactose 2-Deoxy-D-GalaCLose D-lyxo-Hexose,2-deoxy 2-Deoxy-D-Galactopyranose 2-Deoxy-D-galactose MFCD00014649 DEOXY-D-GALACTOSE,2 2-deoxy-D-talose (3R,4R,5R)-3,4,5,6-Tetrahydroxyhexanal |
| Description | 2-Deoxy-D-galactose is a glucose analog. 2-Deoxy-D-galactose inhibits glycolysis to inhibits tumor growth. 2-Deoxy-D-galactose is a substance interfering with the fucosylation of glycomacromolecules and impairing memory consolidation in various learning tasks. 2-Deoxy-d-galactose hinders glycoprotein fucosylation in vivo[1]. |
|---|---|
| Related Catalog | |
| In Vitro | 2-Deoxy-D-galactose (1 mM/L; 5 h) is rapid phosphorylation during the first 30 min and decreases to approximately 20% of this rate during the subsequent hours in ascites hepatoma cells[4]. |
| In Vivo | 2-Deoxy-D-galactose (380 mg/kg; i.p.; for 6 times) strongly decreases contents of UMP, UDPG, and UDP galactose in rat livers[1]. 2-Deoxy-D-galactose (2-8 μM; intracerebroventricularly injection; once) shows PAR impairment 30 min before the acquisition trial a dose of 4 μM and 15 min delay after do-gal administration[3]. Animal Model: Male adult Wistar rats with passive avoidance response (PAR) acquisition trial[3] Dosage: 2, 4 and 8 μM Administration: Intracerebroventricularly injection; 2-8 μM; once Result: Exhibited PAR disruption at a dose of 4 μM. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 456.7±45.0 °C at 760 mmHg |
| Melting Point | 107-110 °C(lit.) |
| Molecular Formula | C6H12O5 |
| Molecular Weight | 164.156 |
| Flash Point | 244.1±25.2 °C |
| Exact Mass | 164.068466 |
| PSA | 90.15000 |
| LogP | -3.07 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.534 |
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | 20/21/22-36/37/38 |
| Safety Phrases | S24/25 |
| WGK Germany | 3 |
| HS Code | 2912491000 |
|
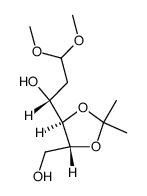
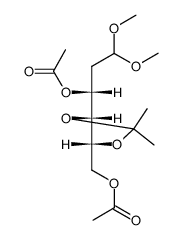
~84% 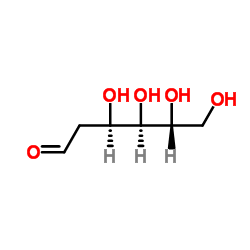
1949-89-9 |
| Literature: Wong, Margaret Y. H.; Gray, Gary R. Carbohydrate Research, 1980 , vol. 80, p. 87 - 98 |
|
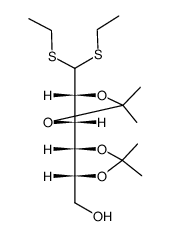
~% 
1949-89-9 |
| Literature: Carbohydrate Research, , vol. 80, p. 87 - 98 |
|
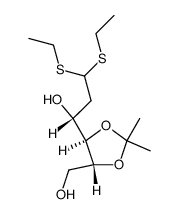
~% 
1949-89-9 |
| Literature: Carbohydrate Research, , vol. 80, p. 87 - 98 |
|
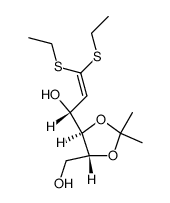
~% 
1949-89-9 |
| Literature: Carbohydrate Research, , vol. 80, p. 87 - 98 |
|
~% 
1949-89-9 |
| Literature: Carbohydrate Research, , vol. 80, p. 87 - 98 |
| Precursor 5 | |
|---|---|
| DownStream 1 | |
| HS Code | 2912491000 |
|---|---|
| Summary | 2912491000. other aldehyde-alcohols. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |