178101-88-7
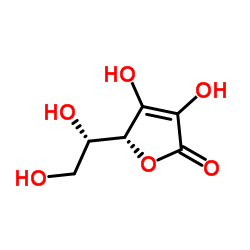
| Name | (5R)-5-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxy-2(5H)-(2-13C)furanone (non-preferred name) |
|---|
| Description | L-Ascorbic acid-13C (L-Ascorbate-13C) is the 13C-labeled L-Ascorbic acid. L-Ascorbic acid (L-Ascorbate), an electron donor, is an endogenous antioxidant agent. L-Ascorbic acid inhibits selectively Cav3.2 channels with an IC50 of 6.5 μM. L-Ascorbic acid is also a collagen deposition enhancer and an elastogenesis inhibitor[1][2][3]. L-Ascorbic acid exhibits anti-cancer effects through the generation of reactive oxygen species (ROS) and selective damage to cancer cells[4]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Molecular Formula | C513CH8O6 |
| Molecular Weight | 177.117 |
| Exact Mass | 177.035446 |
| Index of Refraction | 1.711 |