431979-47-4
| Name | SJ-172550 |
|---|---|
| Synonyms |
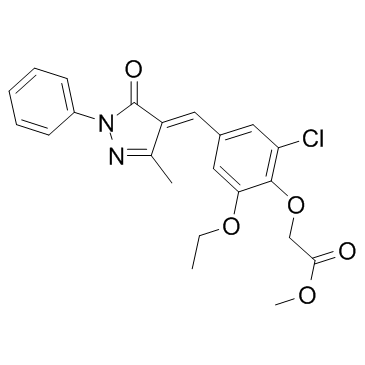
Methyl {2-chloro-6-ethoxy-4-[(Z)-(3-methyl-5-oxo-1-phenyl-1,5-dihydro-4H-pyrazol-4-ylidene)methyl]phenoxy}acetate
Acetic acid, 2-[2-chloro-4-[(Z)-(1,5-dihydro-3-methyl-5-oxo-1-phenyl-4H-pyrazol-4-ylidene)methyl]-6-ethoxyphenoxy]-, methyl ester MFCD03142926 |
| Description | SJ-172550 is a small molecule inhibitor of MDMX; competes for the wild type p53 peptide binding to MDMX with an EC50 of 5 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 5 μM (MDMX)[1] |
| In Vitro | The p53 pathway is disrupted in virtually every human tumor. SJ-172550 binds the p53-binding pocket of MDMX, thereby displacing p53. SJ-172550 binds reversibly to MDMX and effectively kills retinoblastoma cells in which the expression of MDMX is amplified. The effect of SJ-172550 is additive when combined with an MDM2 inhibitor nutlin-3a[1]. SJ-172550 acts through a complicated mechanism in which the compound forms a covalent but reversible complex with MDMX and locks MDMX into a conformation that is unable to bind p53. The relative stability of this complex is influenced by many factors including the reducing potential of the media, the presence of aggregates[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 560.8±60.0 °C at 760 mmHg |
| Molecular Formula | C22H21ClN2O5 |
| Molecular Weight | 428.87 |
| Flash Point | 293.0±32.9 °C |
| Exact Mass | 428.113892 |
| LogP | 3.73 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.586 |
| Storage condition | Store at +4°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |