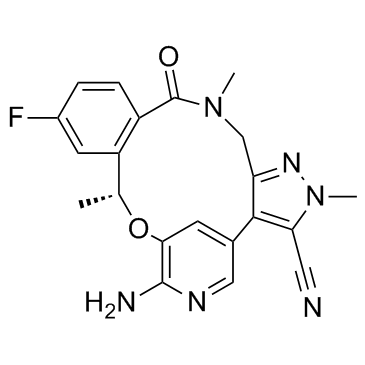
1454846-35-5
| Name | pf-06463922 |
|---|---|
| Synonyms |
UNII:OSP71S83EU
(R)-2<sup>6</sup>-amino-5<sup>5</sup>-fluoro-1<sup>1</sup>,4,7-trimethyl-6-oxo-1<sup>1</sup>H-3-oxa-7-aza-2(3,5)-pyridina-1(4,3)-pyrazola-5(1,2)-benzenacyclooctaphane-1<sup>5</sup>-carbonitrile (16R)-19-Amino-13-fluoro-4,8,16-trimethyl-9-oxo-17-oxa-4,5,8,20-tetraazatetracyclo[16.3.1.0.0]docosa-1(22),2,5,10,12,14,18,20-octaene-3-carbonitrile lorlatinib (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-4,8- methenopyrazolo[4,3-h][2,5,11]benzoxadiazacyclotetradecine-3-carbonitrile PF06463922 PF-06463922 |
| Description | Lorlatinib is a potent, dual ALK/ROS1 inhibitor, with Kis of 0.02 nM, 0.07 nM, and 0.7 nM for ROS1, wild type ALK, and ALK-L1196M, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: < 0.02 nM (ROS1), < 0.07 nM (ALK WT), 0.7 nM (ALK L1196M) |
| In Vitro | Lorlatinib demonstrates significant cell activity against ALK and a large set of ALK clinical mutations with IC50 ranging from 0.2 nM-77 nM[1]. Lorlatinib significantly inhibits cell proliferation and induces cell apoptosis in the HCC78 human NSCLC cells harboring SLC34A2-ROS1 fusions and the BaF3-CD74-ROS1 cells expressing human CD74-ROS1[2]. Lorlatinib also shows potent growth inhibitory activity and induces apoptosis in the NSCLC cells harboring either non-mutant ALK or mutant ALK fusions[3]. |
| In Vivo | In rats, Lorlatinib displays low plasma clearance, a moderate volume of distribution, a reasonable half-life, low propensity for p-glycoprotein 1-mediated efflux and a bioavailability of 100%[1]. In vivo, Lorlatinib shows cytoreductive antitumor efficacy in the NIH3T3 xenograft models expressing human CD74-ROS1 and Fig-ROS1 via inhibition in ROS1 phosphorylation and the downstream signaling molecules, as well as inhibition of the cell cycle protein Cyclin D1 in tumors[2]. In vivo, Lorlatinib also demonstrates marked antitumor activity in mice bearing tumor xenografts expressing EML4-ALK, EML4-ALK-L1196M, EML4-ALK-G1269A, EML4-ALK-G1202R or NPM-ALK[3]. |
| Kinase Assay | Recombinant human wild-type and mutant ALK kinase domain proteins (amino acids 1093–1411) are produced in-house using baculoviral expression, preactivated via autophosphorylation with MgATP, and assayed for kinase activity using a microfluidic mobility shift assay. The reactions contained 1.3 nM wild-type ALK or 0.5 nM mutant ALK (appropriate to produce 15-20% phosphorylation of peptide substrate after 1 h of reaction), 3 μM 5-FAM-KKSRGDYMTMQIG-CONH2), 5 mM MgCl2, and the Km level of ATP in 25 mM Hepes, pH 7.1. The inhibitors are shown to be ATP-competitive from kinetic and crystallographic studies. The Ki values are calculated by fitting the conversion (%) to a competitive inhibition equation. ROS1 enzyme is assayed as described above for ALK, except using 0.25 nM recombinant human ROS1 catalytic domain (amino acids 1883-2347). Kinase inhibitor selectivity is evaluated using a 206-kinase panel. |
| Cell Assay | Cells are seeded in 96-well plates in growth medium containing 10% FBS and are cultured overnight at 37°C. The following day, serial dilutions of Lorlatinib or appropriate controls are added to the designated wells, and cells are incubated at 37°C for 72 h. A CellTiter-Glo assay is performed to determine the relative cell numbers. IC50 values are calculated by concentration-response curve fitting using a four-parameter analytical method. |
| Animal Admin | De novoGBM tumorigenesis is initiated in LSL-FIG-ROS1;Cdkn2a−/−;LSL-Luc mice through intracranial stereotactic injections of Adeno-Cre as described previously. Tumor development is monitored using BLI as described below. Once tumors reach a given size (107 p-1·s-1·cm-2·sr-1), animals are randomLy enrolled into vehicle control or 3-, 7-, or 14-d treatment with the indicated doses of Lorlatinib. Drug is administered through s.c. implanted Alzet osmotic pumps. After treatment, mice are killed, GBM tumors are microdissected, and tissues are flash-frozen in liquid N2. The remaining brains are processed for histology. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 675.0±55.0 °C at 760 mmHg |
| Molecular Formula | C21H19FN6O2 |
| Molecular Weight | 406.413 |
| Flash Point | 362.1±31.5 °C |
| Exact Mass | 406.155365 |
| PSA | 110.06000 |
| LogP | 1.24 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.687 |
| Storage condition | -20℃ |
| RIDADR | NONH for all modes of transport |
|---|