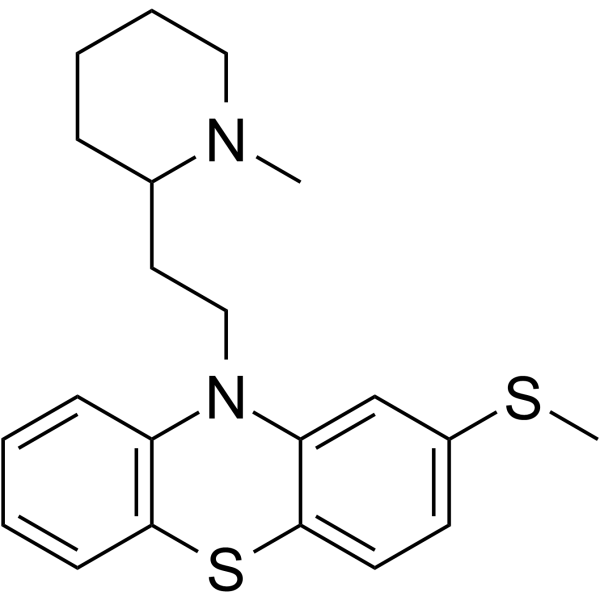
5588-33-0
| Name | mesoridazine |
|---|---|
| Synonyms |
Mesoridazina
Thioridazine-2-sulfoxide Lidanar 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfinylphenothiazine Tps23 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-(methylsulfinyl)-10H-phenothiazine Mesoridazinum 10-[2-(1-methyl-2-piperidyl)ethyl]-2-methylsulphinylphenothiazine Lidanil Calodal Serentil Thioridazine thiomethyl sulfoxide 10-{2-[(RS)1-methylpiperidin-2-yl]ethyl}-2-methylsulfinyl-10H-phenothiazine |
| Description | Mesoridazine (TPS-23) , a metabolite of Thioridazine (HY-B0965A), acts as an orally active phenothiazine antipsychotic agent. Mesoridazine is a potent and rapid open-channel blocker of human ether-a-go-go related gene (hERG) channels and blocks hERG currents with an IC50 of 550 nM (at 0 mV) in human embryonic kidney 293 cells[1].Mesoridazine can be used for the research of schizophrenia, as well as certain other psychiatric disorders[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: hERG currents[1] |
| In Vitro | Mesoridazine blocks human ether-a-go-go-related gene (HERG) currents in a concentration-dependent manner (IC50 = 550 nM at 0 mV), block increased significantly over the voltage range where HERG activates and saturates at voltages eliciting maximal HERG channel activation[1]. Mesoridazine (15 mM; 24 h) shows total absorption of 15.94 ± 4.04% and 39.24 ± 5.11% in nude mouse and pig skin, respectively[3]. |
| In Vivo | Mesoridazine (15 mM; topical administration; once or daily for 7 consecutive days) displays potent activity and a long period of analgesia at blocking cutaneous pain[3]. Mesoridazine (15 mM) shows intradermal concentration of 0.34 0.74 nmol/mg after topical application on nude mouse back for 6 h[3]. Animal Model: Eight-week-old female nude mice[3] Dosage: 15 mM Administration: Topical administration, once (analgesia test) or daily for 7 consecutive days (irritation test) Result: Showed analgesic effect. A slight transepidermal water loss (TEWL) increased from 7.8 to 9.9 g/m2/h was observed. |
| References |
| Density | 1.3g/cm3 |
|---|---|
| Boiling Point | 570.5ºC at 760mmHg |
| Molecular Formula | C21H26N2OS2 |
| Molecular Weight | 386.57400 |
| Flash Point | 298.9ºC |
| Exact Mass | 386.14900 |
| PSA | 68.06000 |
| LogP | 5.76970 |
| Index of Refraction | 1.694 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~92% 
5588-33-0 |
| Literature: Morrow, Ryan J.; Millership, Jeff S.; Collier, Paul S. Helvetica Chimica Acta, 2005 , vol. 88, # 5 p. 962 - 967 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |