174634-08-3
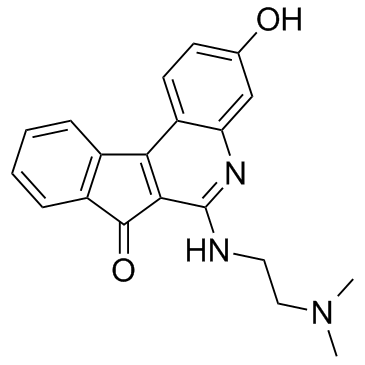
| Name | 3-hydroxy-6-(2-dimethylaminoethylamino)indeno[2,1-c]quinolin-7-one |
|---|---|
| Synonyms |
7H-Indeno[2,1-c]quinolin-7-one, 6-[[2-(dimethylamino)ethyl]amino]-3-hydroxy-
6-{[2-(Dimethylamino)ethyl]amino}-3-hydroxy-7H-indeno[2,1-c]quinolin-7-one TAS 103 6-[[2-(dimethylamino)-ethyl]amino]-3-hydroxy-7H-indeno[2,1-c]quinolin-7-one 6-[[2-(dimethylamino)ethyl]amino]-3-hydroxy-7H-indeno[2,1-c]quinolin-7-one TAS-103 |
| Description | TAS-103 is a dual inhibitor of DNA topoisomerase I/II, used for cancer research. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I Topoisomerase II |
| In Vitro | TAS-103 is a dual inhibitor of DNA topoisomerase I/II. TAS-103 (0.1-10 μM) is active on CCRF-CEM cells, with an IC50 value of 5 nM. TAS-103 (0.1 μM) significantly increases levels of topo IIα FITC immunofluorescence in individual CCRF-CEM cells[1]. TAS-103 (0.01-1 μM) is highly cytotoxic to Lewis lung carcinoma (LLC) cells, and Liposomal TAS-103 is almost as active as free TAS-103[2]. TAS-103 inhibits the viability of HeLa cells, with an IC50 of 40 nM. TAS-103 (10 μM) disrupts signal recognition particle (SRP) complex formation, and induces destabilization of SRP14 and SRP19 and its eventual degradation[3]. |
| In Vivo | TAS-103 (30 mg/kg, i.v.) causes significant tumor growth suppression in mice bearing Lewis lung carcinoma (LLC) cells, without obvious body weight loss, and the liposomal TAS-103 is more active than free TAS-103[2]. |
| Cell Assay | CCRF-CEM human acute lymphoblastic leukaemia cells are grown in RPMI-1640 supplemented with 3 mM l-glutamine, 10% foetal bovine serum, 50 U/mL of penicillin, and 40 μg/mL of streptomycin at 37°C in a humidified atmosphere containing 5% CO2. TAS-103, CPT and DACA are dissolved in DMSO. Exponentially growing cells (∼5 × 105) are exposed to either of the drugs for 2 hrs. Following drug exposure, cells are washed twice by centrifugation (400 × g, 3 min) in cold phosphate-buffered saline[1]. |
| Animal Admin | Lewislung carcinoma (LLC) cells are diluted with DMEM to obtain 5×106 cells/mL suspension, and 0.2 mL of the suspension is carefully injected subcutaneously into five-week-old C57BL/6 male mice. Liposomal TAS-103 (0.2 mL/mouse, 30 mg/kg as TAS-103), free TAS-103 or PBS is injected intravenously into a tail vein of the tumor-bearing mice on days 4, 8, and 12 after tumor implantation. Tumor volume of each mouse and the body weight change as an indicator of side effect are monitored daily thereafter. Tumor volume is calculated[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 589.5±50.0 °C at 760 mmHg |
| Molecular Formula | C20H19N3O2 |
| Molecular Weight | 333.384 |
| Flash Point | 310.3±30.1 °C |
| Exact Mass | 333.147736 |
| PSA | 65.46000 |
| LogP | 2.71 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.734 |
| Storage condition | 2-8℃ |