3943-89-3
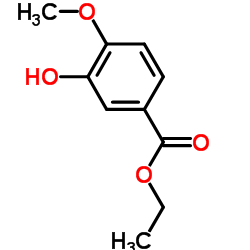
| Name | Ethyl 3,4-dihydroxybenzoate |
|---|---|
| Synonyms |
3,4-dihydroxybenzoic acid ethyl ether
Ethyl-3,4-dihydroxybenzoate Benzoic acid, 3,4-dihydroxy-, ethyl ester Ethyl protocatechuate EDHB 3,4-Dihydroxybenzoic acid ethyl ester EINECS 223-529-0 Benzoic acid, 3,4-dihydroxy-, ethyl ester (9CI) Protocatechuic acid ethyl ester MFCD00002199 Ethyl 3,4-dihydroxybenzoate |
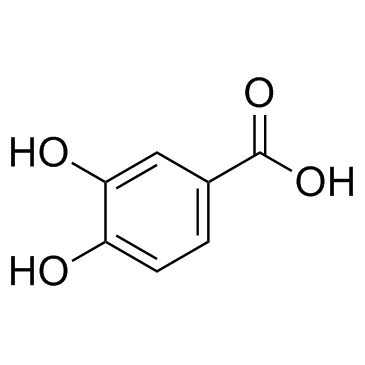
| Description | Ethyl 3,4-dihydroxybenzoate (Ethyl protocatechuate), an antioxidant, is a prolyl-hydroxylase inhibitor found in the testa of peanut seeds. Ethyl 3,4-dihydroxybenzoate protects myocardium by activating NO synthase and generating mitochondrial ROS. Ethyl 3,4-dihydroxybenzoate induces cell autophagy and apoptosis in ESCC cells. Ethyl 3,4-dihydroxybenzoate is a collagen synthesis inhibitor and has a bone protecting-effect[1][2][3][4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 358.1±22.0 °C at 760 mmHg |
| Melting Point | 132-134 °C(lit.) |
| Molecular Formula | C9H10O4 |
| Molecular Weight | 182.173 |
| Flash Point | 147.0±15.8 °C |
| Exact Mass | 182.057907 |
| PSA | 66.76000 |
| LogP | 2.22 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.574 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2918290000 |
|
~99% 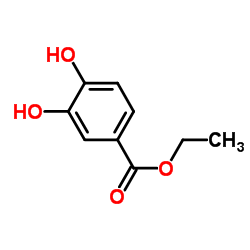
3943-89-3 |
| Literature: Rey, Jimena; Garcia, Felix Clemente; Garcia, Jose Miguel Reactive and Functional Polymers, 2011 , vol. 71, # 9 p. 948 - 957 |
|
~78% 
3943-89-3 |
| Literature: Huang, Chunhui; Ghavtadze, Nugzar; Chattopadhyay, Buddhadeb; Gevorgyan, Vladimir Journal of the American Chemical Society, 2011 , vol. 133, # 44 p. 17630 - 17633 |
|
~90% 
3943-89-3 |
| Literature: Huang, Wei-Bin; Guo, Ying; Jiang, Jian-An; Pan, Xian-Dao; Liao, Dao-Hua; Ji, Ya-Fei Synlett, 2013 , vol. 24, # 6 art. no. ST-2013-W0052-L, p. 741 - 746 |
|
~% 
3943-89-3 |
| Literature: Huang, Chunhui; Ghavtadze, Nugzar; Chattopadhyay, Buddhadeb; Gevorgyan, Vladimir Journal of the American Chemical Society, 2011 , vol. 133, # 44 p. 17630 - 17633 |
|
~% 
3943-89-3 |
| Literature: Matsmoto Chemische Berichte, 1878 , vol. 11, p. 133 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2918290000 |
|---|---|
| Summary | HS: 2918290000 other carboxylic acids with phenol function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) VAT:17.0% MFN tariff:6.5% General tariff:30.0% |









![ETHYL 2,3-DIHYDROBENZO[B][1,4]DIOXINE-6-CARBOXYLATE structure](https://image.chemsrc.com/caspic/122/20825-87-0.png)