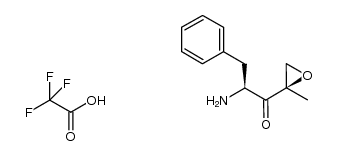
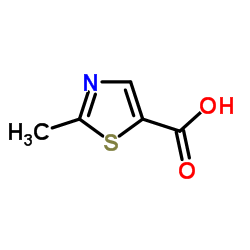
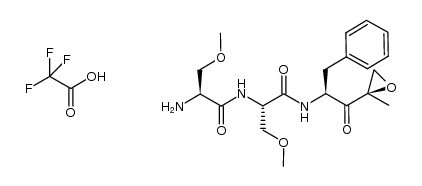
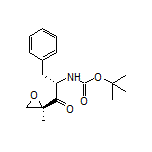
935888-69-0
| Name | N-[(2S)-3-methoxy-1-[[(2S)-3-methoxy-1-[[(2S)-1-[(2R)-2-methyloxiran-2-yl]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]-2-methyl-1,3-thiazole-5-carboxamide |
|---|---|
| Synonyms |
ONX-0912
4,5-Anhydro-1,2-dideoxy-4-methyl-2-({O-methyl-N-[(2-methyl-1,3-thiazol-5-yl)carbonyl]-L-seryl-O-methyl-L-seryl}amino)-1-phenyl-D-erythro-pent-3-ulose QC-9273 PR-047 ONX 0912 D-erythro-3-Pentulose, 4,5-anhydro-1,2-dideoxy-4-C-methyl-2-[[O-methyl-N-[(2-methyl-5-thiazolyl)carbonyl]-L-seryl-O-methyl-L-seryl]amino]-1-phenyl- |
| Description | Oprozomib (ONX 0912; PR047) is an orally bioavailable inhibitor for CT-L activity of 20S proteasome β5/LMP7 with IC50 of 36 nM/82 nM.IC50 value: 36 nM/82 nM(20S proteasome β5/LMP7) [1]Target: 20S proteasomeThe anti-MM activity of Oprozomib is associated with activation of caspase-8, caspase-9, caspase-3, and PARP, as well as inhibition of migration of MM cells and angiogenesis. Oprozomib is demonstrated an absolute bioavailability of up to 39% in rodents and dogs. It is well tolerated with repeated oral administration at doses resulting in >80% proteasome inhibition in most tissues and elicited an antitumor response in multiple human tumor xenograft and mouse syngeneic models. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 849.9±65.0 °C at 760 mmHg |
| Molecular Formula | C25H32N4O7S |
| Molecular Weight | 532.609 |
| Flash Point | 467.8±34.3 °C |
| Exact Mass | 532.199158 |
| PSA | 186.96000 |
| LogP | 2.79 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.573 |
| Storage condition | -20℃ |
|
~86% 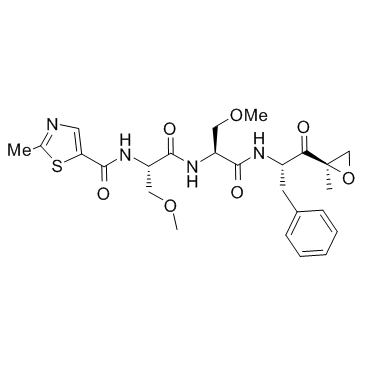
935888-69-0 |
| Literature: Jumaa, Mouhannad; Muchamuel, Tony; Bejugam, Naveen; Wong, Hansen; Kirk, Christopher J.; Vishram Manek, Rahul; Sharma, Sanjeev Patent: US2014/113855 A1, 2014 ; Location in patent: Paragraph 0279; 0305; 0306; 0307; 0308 ; |
|
~% 
935888-69-0 |
| Literature: Zhou, Han-Jie; Aujay, Monette A.; Bennett, Mark K.; Dajee, Maya; Demo, Susan D.; Fang, Ying; Ho, Mark N.; Jiang, Jing; Kirk, Christopher J.; Laidig, Guy J.; Lewis, Evan R.; Lu, Yan; Muchamuel, Tony; Parlati, Francesco; Ring, Eileen; Shenk, Kevin D.; Shields, Jamie; Shwonek, Peter J.; Stanton, Timothy; Sun, Congcong M.; Sylvain, Catherine; Woo, Tina M.; Yang, Jinfu Journal of Medicinal Chemistry, 2009 , vol. 52, # 9 p. 3028 - 3038 |
|
~% 
935888-69-0 |
| Literature: US2014/113855 A1, ; |
|
~% 
935888-69-0 |
| Literature: US2014/113855 A1, ; |
|
~% 
935888-69-0 |
| Literature: US2014/113855 A1, ; |
|
~% 
935888-69-0 |
| Literature: US2014/113855 A1, ; |
|
~% 
935888-69-0 |
| Literature: US2014/113855 A1, ; |
| Precursor 4 | |
|---|---|
| DownStream 0 | |