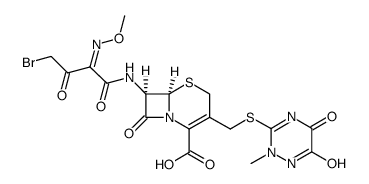
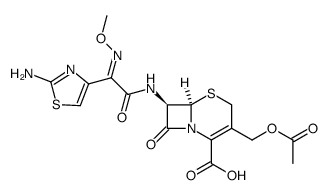
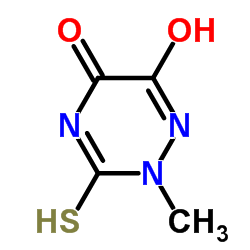
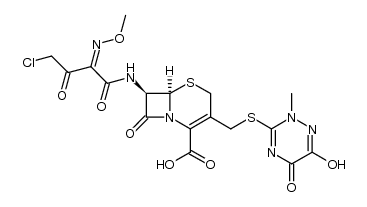
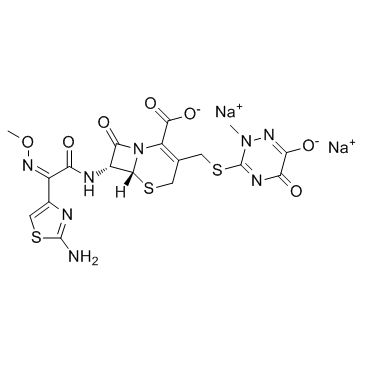
73384-59-5
| Name | ceftriaxone |
|---|---|
| Synonyms |
Ceftriazone
Biotrakson Rocefin (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-3-{[(6-hydroxy-2-methyl-5-oxo-2,5-dihydro-1,2,4-triazin-3-yl)thio]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Ceftriaxone (6R,7R)-7-{(E)-[(2Z)-1-Hydroxy-2-(2-imino-2,3-dihydro-1,3-thiazol-4-yl)-2-(methoxyimino)ethylidene]amino}-3-{[(2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Ro 13-9904/001 ceftriaxonum [INN_la] ceftriaxome (6R,7R)-7-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-{[(6-hydroxy-2-methyl-5-oxo-2,5-dihydro-1,2,4-triazin-3-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Ceftriaxone [USAN:JAN] Antibiotic Ro-13-9904 Cefatriaxone (6R,7R)-7-[[(2E)-2-(2-Amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(2-methyl-5,6-dioxo-1H-1,2,4-triazin-3-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid MFCD00865013 EINECS 277-405-6 (6R,7R)-7-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-{[(2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Rocephin |
| Description | Ceftriaxone is an antibiotic useful for the treatment of a number of bacterial infections.Target: AntibacterialCeftriaxone inhibits bacterial cell wall synthesis by means of binding to the penicillin-binding proteins (PBPs). Inhibition of PBPs would in turn inhibit the transpeptidation step in peptidoglycan synthesis which is required for bacterial cell walls. Like other cephalosporins, ceftriaxone is bacteriocidal and exhibits time-dependent killing. Ceftriaxone, one of the beta-lactam antibiotics, is a stimulator of EAAT2 expression with neuroprotective effects in both in vitro and in vivo models based in part on its ability to inhibit neuronal cell death by glutamate excitotoxicity. Based on this consideration and its lack of toxicity, ceftriaxone has potential to manipulate glutamate transmission and ameliorate neurotoxicity [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Melting Point | 155 °C |
| Molecular Formula | C18H18N8O7S3 |
| Molecular Weight | 554.580 |
| Exact Mass | 554.046082 |
| PSA | 293.80000 |
| LogP | -0.77 |
| Index of Refraction | 1.889 |
| Storage condition | 2-8℃ |
|
~53% 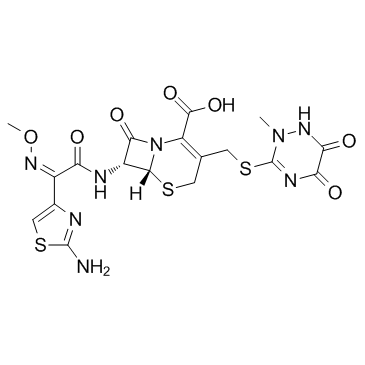
73384-59-5 |
| Literature: LUPIN LIMITED Patent: WO2004/58695 A1, 2004 ; Location in patent: Page 29 ; |
|
~% 
73384-59-5 |
| Literature: WO2005/105813 A1, ; Page/Page column 8 ; |
|
~% 
73384-59-5 |
| Literature: US2002/128469 A1, ; |
|
~% 
73384-59-5 |
| Literature: WO2004/111059 A1, ; Page/Page column 6-8 ; |
| Precursor 3 | |
|---|---|
| DownStream 1 | |
| HS Code | 3003201700 |
|---|