124832-26-4
| Name | valacyclovir |
|---|---|
| Synonyms |
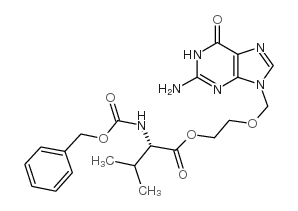
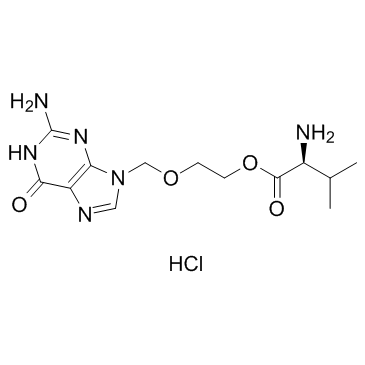
2-{[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methyl]oxy}ethyl L-valinate
L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester L-Valine 2-(guanin-9-ylmethoxy)ethyl ester Valacyclovir Valtrex L-valine ester with 9-[(2-hydroxyethoxy)methyl]guanine 2-[(2-amino-6-hydroxy-9H-purin-9-yl)methoxy]ethyl L-valinate L-valine, 2-[(2-amino-6-hydroxy-9H-purin-9-yl)methoxy]ethyl ester Valaciclovir L-Valine 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate |
| Description | Valacyclovir is an antiviral drug used in the management of herpes simplex, herpes zoster, and herpes B. IC50 Value: 2.9 microg/ml (for HSV-1 W)[4]. Target: HSV infectionin vitro: VACV uptake was concentration dependent and saturable with a Michaelis-Menten constant and maximum velocity of 1.64 +/- 0.06 mM and 23.34 +/- 0.36 nmol/mg protein/5 min, respectively. A very similar Km value was obtained in hPEPT1/CHO cells and in rat and rabbit tissues and Caco-2 cells, suggesting that hPEPT1 dominates the intestinal transport properties of VACV in vitro [5]. in vivo: For treatment of a first episode of genital herpes, a large comparative trial has shown that valacyclovir (1 g twice a day) is as effective as acyclovir (200 mg five times a day) when given for 10 days. For treating recurrences, two trials show that valacyclovir is as effective as acyclovir (200 mg five times a day) with a treatment period of 5 days. A daily dose of 1 g of valacyclovir is as effective as 2 g daily. Valacyclovir can be administered once a day[1]. The concentrations of acyclovir in serum and CSF were measured at steady state after 6 days of oral treatment with 1,000 mg of valacyclovir three times a day [2]. EC50 values of PE and AC in 3T3 cells were 0.02 and 0.01 ug/ml, while values in BHK cells were 0.2 and 0.03 ug/ml. Treatment of infected immunosuppressed mice and FA and VA (b.i.d., 5.5 days) reduced the proportion with erythema from 100% to 24% and 38%, and eliminated ear paralysis, ear lesions (vesicles, etc) and death. Virus was absent from ear and brainstem by day 6, but reappeared after discontinuation in mice treated with VA [3].Clinical trial: Evaluation of Valaciclovir in Patients with Chickenpox. Phage3 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C13H20N6O4 |
| Molecular Weight | 324.336 |
| Flash Point | 309.7ºC |
| Exact Mass | 324.154602 |
| PSA | 151.14000 |
| LogP | -0.88 |
| Index of Refraction | 1.673 |
| Storage condition | 2-8℃ |
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| HS Code | 3002909099 |
|
~% 
124832-26-4 |
| Literature: US4957924 A1, ; |
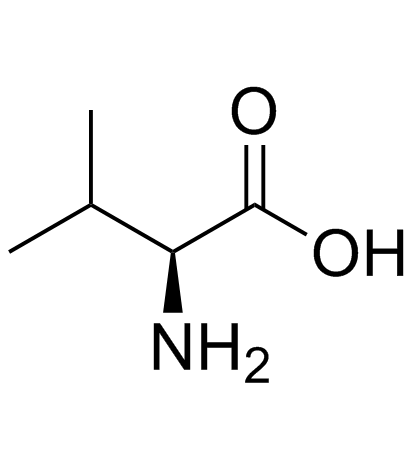
| Precursor 1 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
![2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]ethyl (2S)-2-formamido-3-methylbutanoate structure](https://image.chemsrc.com/caspic/062/847670-62-6.png)