321-02-8
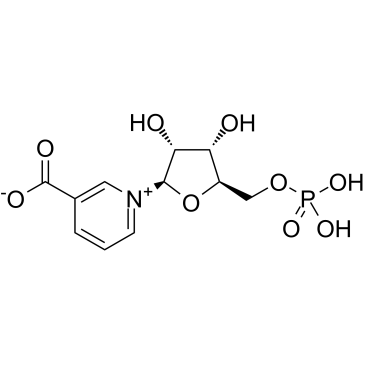
| Name | 3-Carboxy-1-(5-O-phosphono-β-D-ribofuranosyl)pyridinium |
|---|---|
| Synonyms |
3-Carboxy-1-methylpyridiniumiodid
Pyridinium,3-carboxy-1-methyl-,iodide Trigonelline hydriodide trigonelline hydroiodide nicotinic acid mononucleotide Nicotinsaeure-iodmethylat Nicotinsaeure-mononucleotid nicotinic acid methiodide nicotinate adenine mononucleotide 3-Carboxy-1-methyl-pyridinium,Jodid 3-carboxy-1-methyl-pyridinium,iodide |
| Description | Nicotinic acid mononucleotide (NAMN) is formed from nicotinic acid (NA) via the nicotinic acid phosphoribosyltransferase in the biosynthesis of NAD+. Nicotinate mononucleotide is a substrate for nicotinamide mononucleotide/Nicotinic acid mononucleotide adenylyltransferase[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C11H14NO9P |
|---|---|
| Molecular Weight | 336.21200 |
| Exact Mass | 336.04800 |
| PSA | 167.44000 |
| Storage condition | 20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |