749886-87-1
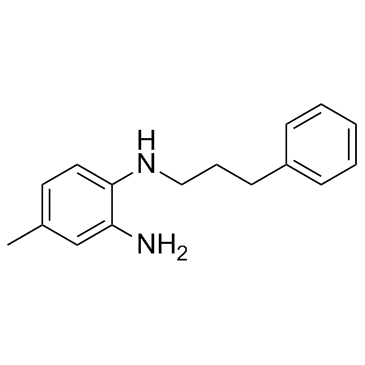
| Name | 4-methyl-1-N-(3-phenylpropyl)benzene-1,2-diamine |
|---|---|
| Synonyms |
4-Methyl-N-(3-phenylpropyl)-1,2-benzenediamine
IN1201 JSH-23 FD5025 NF-kappaB Activation Inhibitor II,JSH-23 |
| Description | JSH-23 is an NF-κB inhibitor which inhibits NF-κB transcriptional activity with an IC50 of 7.1 μM. |
|---|---|
| Related Catalog | |
| Target |
NF-κB:7.1 μM (IC50, in RAW 264.7 cells) |
| In Vitro | JSH-23 inhibits lipopolysaccharide (LPS)-induced chromatin condensation in a dose-dependent manner, corresponding to 44±4% inhibition at 3 μM, 63±5% at 10 μM and 93±3% at 30 μM[1]. JSH-23 (5, 10, and 15 μM) significantly reduces mean neuronal migration in LPS-activated cells[2]. Co-treatment of A2780 cells with JSH-23 and clinically ineffective transplatin at their IC50 concentrations (130 μM transplatin and 20 μM JSH-23) for 72 h also causes a more pronounced decrease in cell viability compared to the effects of transplatin or JSH-23 alone[3]. |
| In Vivo | JSH-23 (1 and 3 mg/kg, p.o.) significantly reverses the nerve conduction and nerve blood flow deficits seen in diabetic animals and decreases the nerve lipid peroxidation, partially replenishes the depleted levels of GSH in nerve of diabetic rats[4]. |
| Cell Assay | Apoptosis is analyzed by 4',6-diamidino-2-phenylindole (DAPI) staining. Macrophages RAW 264.7 incubated with JSH-23 are treated with 1 μg/mL LPS and/or sample for 24 h. The cells are stained with 1 μg/mL DAPI for 30 min at 37°C and then analyzed using fluorescence microscopy with excitation at 300-500 nm. Cells with nuclei containing clearly condensed chromatin or cells with fragmented nuclei are scored as an apoptosis index. |
| Animal Admin | Male Sprague Dawley rats (250-270 g) are used and fed on standard rat diet and water ad libitum. Diabetes is induced by single dose of streptozotocin (STZ, 55 mg/kg, intraperitoneally) in citrate buffer. Blood samples are collected 48 h after STZ administration. Rats with plasma glucose level more than 250 mg/dL are considered as diabetics and are further considered for study. The experimental groups comprised of non-diabetic control rats (ND), diabetic control rats (STZ-D) and diabetic rats treated with two doses of JSH-23 (STZ-D + JSH 1 and STZ-D + JSH 3, respectively, for 1 and 3 mg/kg, orally in 0.5% sodium carboxymethyl cellulose). After 6 weeks of diabetes induction, the drug is administered daily for a period of 2 weeks. The functional, behavioural and biochemical experiments are performed 24 h after the administration of last dose. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 418.7±40.0 °C at 760 mmHg |
| Melting Point | 104.4-105.0℃ |
| Molecular Formula | C16H20N2 |
| Molecular Weight | 240.343 |
| Flash Point | 245.0±30.9 °C |
| Exact Mass | 240.162643 |
| PSA | 38.05000 |
| LogP | 3.66 |
| Appearance | off-white to gray-pink |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.630 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: >10mg/mL |
| Symbol |



GHS05, GHS07, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318-H400 |
| Precautionary Statements | P273-P280-P305 + P351 + P338 |
| Hazard Codes | Xn,N |
| Risk Phrases | 22-41-50/53 |
| Safety Phrases | 26-39-60-61 |
| RIDADR | UN 3077 9 / PGIII |