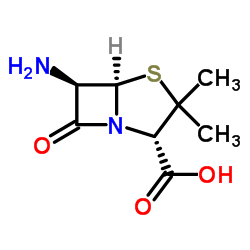
66-79-5
| Name | oxacillin |
|---|---|
| Synonyms |
OXACILLIN
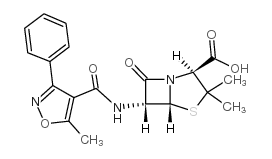
[2S-(2,5,6)]-3,3-dimethyl-6-(methyl-3-phenylisoxaz-4-ole-carboxamide-7-oxy-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Oxazina (2S,5R,6R)-3,3-Dimethyl-6-[(5-methyl-3-phenylisoxazol-4-yl)carbonylamino]-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Penstapho (5-methyl-3-phenyl-isoxazol-4-yl)-penicillin Bactocil oxazocillin |
| Description | Oxacillin is an orally active synthetic penicillin with good bactericidal activity against staphylococci and other gram-positive pathogens[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Oxacillin inhibits gram positive pathogens with MICs of 0.05, 0.09, 0.32 and 0.80 μg/mL against group A streptococci, Pneutnococci, susceptible staphylococci and penicillin-resistant staphylococci, respectively[1]. The Oxacillin-resistant strains are highly resistant to other penicillins[1]. |
| In Vivo | Oxacillin (50-800 mg/kg; s.c.; once) shows curative dose (CD50) of 253.3 mg/kg in mice infected with Staphylococcus aureus Evans. The oral CD50 of Oxacillin is 187.2 mg/kg[2]. Animal Model: CD-1 strain male albino mice infected with S. aureus Evans[2] Dosage: 50, 100, 200, 400 and 800 mg/kg Administration: Subcutaneous injection, once Result: Showed therapeutic activity with CD50 of 253.3 mg/kg. |
| Melting Point | 188ºC |
|---|---|
| Molecular Formula | C19H19N3O5S |
| Molecular Weight | 401.43600 |
| Exact Mass | 401.10500 |
| PSA | 138.04000 |
| LogP | 2.22410 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~% 
66-79-5 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 673, p. 166 - 170 |
|
~% 
66-79-5 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 673, p. 166 - 170 |
| Precursor 3 | |
|---|---|
| DownStream 1 | |