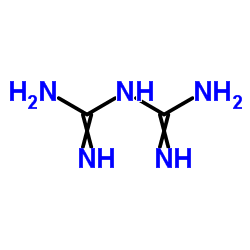
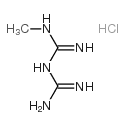
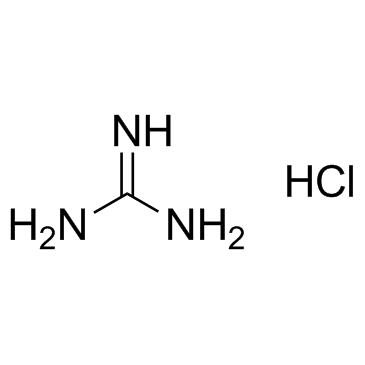
50-01-1
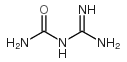
| Name | Guanidine hydrochloride |
|---|---|
| Synonyms |
ZYZUM &&HCl
Guanidine hydrochloride (1:1) guanidinium chloride Guanidine, hydrochloride (1:1) Guanidine monohydrochloride Amino(imino)methanaminium chloride GdmCl Guanidine (hydrochloride) guanidine chlorhydrate hydrochloric acid guanidine Aminoformamidine hydrochloride,Aminomethanamidine hydrochloride,Guanidinium chloride Guanidine Hydrochlor Guanidine chloride Aminoformamidine hydrochloride GUANIDIUM CHLORIDE GUHY Aminoformamidine hydrochloride,Guanidinium chloride,Guanidium chloride 1-methylguanidine hydrochloride Aminomethanamidine hydrochlorid MFCD00013026 AMINOFORMAMIDINE HCL Guanidinehydrochloride Guanidine, monohydrochloride usafek-749 EINECS 200-002-3 Guanidinhydrochlorid Guanidine Hydrochloride Guanidine HCl guanidinium hydrochloride |
| Description | Guanidine HCl, the crystalline compound of strong alkalinity formed by the oxidation of guanine, is a normal product of protein metabolism and a protein denaturant.Target: OthersGuanidine HCl is the most popular protein denaturant. Analysis of unfolding transitions by Guanidine HCl provides several important parameters regarding the mechanism of conformational stability of proteins. Guanidine HCl at low concentrations refolds acid-unfolds apomyoglobin and cytochrome c, stabilizing the molten globule state. Guanidine HCl (> 1 M) causes co-operative unfolding of the molten globule state [1]. Guanidine HCl at millimolar concentrations, is able to causes efficient loss of the normally stable [PSI+] element from yeast cells. 5 mM Guanidine HCl in growth media cures [PSI+] and other prions of yeast. 5 mM Guanidine HCl significantly reduces Hsp104-mediated basal and acquired thermotolerance by 30-fold and 50 fold, respectively. Guanidine HCl also reduces the ability of Hsp104 to restore activity of thermally denatured luciferase [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.18 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 132.9ºC at 760 mmHg |
| Melting Point | 180-185 °C(lit.) |
| Molecular Formula | CH6ClN3 |
| Molecular Weight | 95.531 |
| Exact Mass | 95.025024 |
| PSA | 75.89000 |
| LogP | 1.14090 |
| Index of Refraction | n20/D 1.465 |
| Storage condition | Store at RT. |
| Stability | Stable. Hygroscopic. Incompatible with strong oxidizing agents. |
| Water Solubility | 2280 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H332-H315-H319 |
| Precautionary Statements | P261-P280-P301 + P312 + P330-P304 + P340 + P312-P305 + P351 + P338-P337 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/38 |
| Safety Phrases | S26-S36-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | MF4300000 |
| HS Code | 2925290090 |
|
~% 
50-01-1 |
| Literature: Sander Patent: DE527237 , 1928 ; Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 18, p. 358 |
|
~% 
50-01-1 |
| Literature: Staehler, A. Ber. Dtsch. Chem. Ges., 1914 , vol. 47, p. 903 - 913 |
|
~% 
50-01-1 |
| Literature: Gmelin Handbook: C: MVol.D1, 49.1.2, page 466 - 467 Full Text Show Details Sander, F. Patent: DE527237 , 1931 ; |
|
~% 
50-01-1 |
| Literature: Rathke Chemische Berichte, 1885 , vol. 18, p. 3106 Chemische Berichte, 1887 , vol. 20, p. 1059 |
|
~%
Detail
|
| Literature: Staehler Chemische Berichte, 1914 , vol. 47, p. 912 |
|
~% 
50-01-1 |
| Literature: Knorr Chemische Berichte, 1917 , vol. 50, p. 234 |
|
~% 
50-01-1
Detail
|
| Literature: Sugino; Yamashida Nippon Kagaku Kaishi, 1944 , vol. 65, p. 271,279 Chem.Abstr., 1947 , p. 3763 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2925290090 |
|---|---|
| Summary | 2925290090 other imines and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |