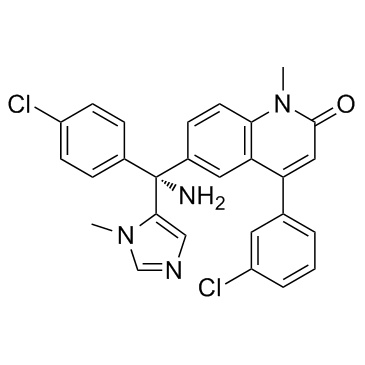
192185-72-1
| Name | 6-[(R)-amino-(4-chlorophenyl)-(3-methylimidazol-4-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2-one |
|---|---|
| Synonyms |
Zarnestra
6-[(R)-Amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone S1453_Selleck tipifarnib Tipifarnib (Zarnestra) 6-[(r)-amino(4-chlorophenyl)(1-methyl-1h-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2(1h)-one |
| Description | Tipifarnib is a potent and specific farnesyltransferase (FTase) inhibitor with IC50 of 0.6 nM, and the anti-proliferative effects are most prominent in H-ras or N-ras mutant cells. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.6 nM (FTase) |
| In Vitro | Tipifarnib (5 μM) leads the percentage of apoptotic cells significantly higher in drug-treated compared to DMSO-treated LGL T-cells. Using T-cells from healthy donors, tipifarnib reduces the percentage of IFNγ-positive cells in a time-dependent manner. Tipifarnib reduces the amount of activated Ras in precipitates compared to DMSO[2]. Tipifarnib exerts selective in vitro toxicity against clonal MDS hematopoiesis at concentrations less than 10 nM the effect being more prominent in white cell progenitors. This action is not due to apoptosis induction as both normal and MDS progenitors displays equivalent DiOC3 and annexin V expression up to 72 hours after exposure to Tipifarnib[3]. Combining Tipifarnib with 10 nM 4-OH-tamoxifen in the presence of E2 reduces the IC50 8-fold from 400 to 50 nM[4]. Tipifarnib induces apoptosis in U937 cells[5]. In addition, Tipifarnib inhibits isolated human farnesyltransferase for a lamin B peptide and for the K-RasB peptide with IC50 of 0.86 nM and 7.9 nM, respectively[6]. |
| In Vivo | Tipifarnib has the light of the modest toxicity in patients and the potent reduction of graft-versus-host disease in mice, and it could help to reduce graft-versus-host disease significantly without having a negative impact on immune reconstitution[1]. Combined therapy with tamoxifen and Tipifarnib (50 mg/kg, p.o.) produces greater tumor growth inhibition when compared with either drug alone. E2 deprivation and Tipifarnib in combination results in greater growth inhibition than either E2 deprivation or Tipifarnib alone. The combination of tamoxifen and Tipifarnib results in significantly lower Ki-67 compared with either tamoxifen or Tipifarnib alone. Tipifarnib alone also reduces the CTI compared with control. The combination of tamoxifen and Tipifarnib or Tipifarnib coupled with E2 withdrawal is most effective at lowering the CTI (0.8 and 0.7, respectively), which may account for the decrease in tumor volume[4]. |
| Cell Assay | Steroid-depleted cells are seeded into 12-well plates at a density of appr 1×104 cells per well or into 96-well plates at a density of 4,000 cells per well, in dextran-coated charcoal medium. After 24 h, monolayers are treated with E2 plus inhibitors either alone or in combination. The 12-well plates are treated for 6 days with daily changes. Cell number is then determined using a Z1 Coulter counter. The 96-well plates are treated with a single dose and left for 96 h at which time cell viability is measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described previously. The interaction between Tipifarnib and 4-OH-tamoxifen is analyzed by the median effect plot method described by Chou and Talalay. Calculation of the combination index took into account a nonfixed drug ratio and is based on the assumption that the action of the two drugs is mutually nonexclusive for the strict detection of synergism. A combination index < 1 indicates synergism, combination index=1 indicates additivity, and a combination index > 1 indicates antagonism. Experiments are repeated thrice. |
| Animal Admin | Female ovariectomized Ncr foxhead nude mice are kept under sterile conditions with free access to food and water. MCF-7 xenografts are initiated by implantation of 2-mm diameter tumor fragments from established tumors. Tumor growth is maintained by E2 supplementation through i.d. injection of 17β-estradiol pellets (dose 1.7 mg over 60 days). Once tumors reach a diameter of appr 7 mm, mice are randomized to receive vehicle [20% w/v β-cyclodextrin (pH 2.5) for Tipifarnib, 50% PEG 300, 50% H2O + 1 drop 1N HCl per 3 mL for tamoxifen], Tipifarnib (50 mg/kg twice daily), tamoxifen (20 mg/kg), or a combination of both Tipifarnib and tamoxifen. Two further treatment arms are used to assess the effect of E2 withdrawal (removal of the E2 pellet) or E2 withdrawal combined with Tipifarnib (50 mg/kg twice daily). All drugs are given by oral gavage daily for 5 consecutive days followed by a 2-day rest period, for a total of 19 days. The experiment is done twice giving similar results; therefore, the growth data are combined for statistical analysis. There are six tumor-bearing animals in each group and all tumors are harvested on day 19. Tumor volumes are calculated using the formula a × b2 × π/6, where a and b are orthogonal tumor diameters and expressed as a percentage of the volume at the start of treatment (relative tumor volume). Overall statistical difference is calculated using the Kruskal-Wallace test and statistical differences between individual treatment arms are calculated using the Mann-Whitney test. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 681.7±55.0 °C at 760 mmHg |
| Melting Point | 211-213ºC (dec.) |
| Molecular Formula | C27H22Cl2N4O |
| Molecular Weight | 489.396 |
| Flash Point | 366.1±31.5 °C |
| Exact Mass | 488.117065 |
| PSA | 65.84000 |
| LogP | 4.94 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.672 |
| Storage condition | -20?C Freezer |