52328-97-9
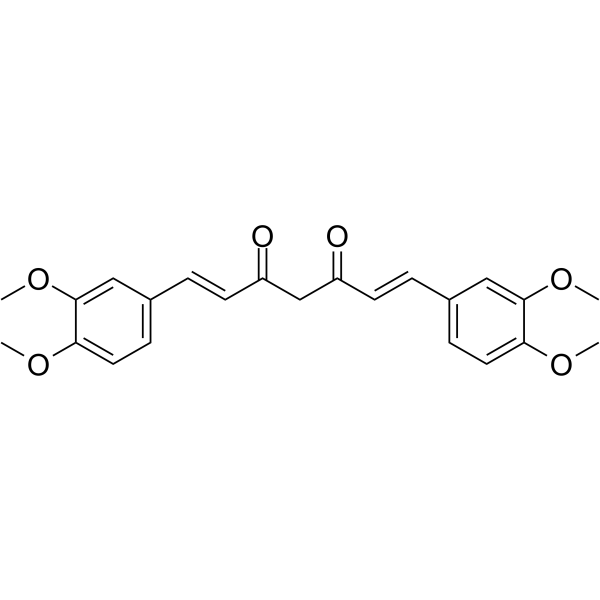
| Name | (1E,6E)-1,7-bis(3,4-dimethoxyphenyl)-4,4-dimethylhepta-1,6-diene-3,5-dione |
|---|---|
| Synonyms |
FLLL31
(E,E)-1,7-Bis(3,4-dimethoxyphenyl)-4,4-dimethyl-1,6-heptadiene-3,5-dione Tetramethylcurcumin (1E,6E)-1,7-Bis(3,4-dimethoxyphenyl)-4,4-dimethyl-1,6-heptadiene-3,5-dione |
| Description | Tetramethylcurcumin (FLLL31), derived from curcumin, specifically suppresses the phosphorylation of STAT3 by binding selectively to Janus kinase 2 and the STAT3 Src homology-2 domain. Tetramethylcurcumin exhibits anti-inflammatory and anti-cancer effects[1][2]. |
|---|---|
| Related Catalog | |
| Target |
STAT3 |
| In Vitro | Tetramethylcurcumin (FLLL31; 2.5 and 5 µM; for 24 hours) downregulats STAT3 phosphorylation and DNA-binding activity in MDA-MB-231 breast and PANC-1 pancreatic cancer cells[1]. Tetramethylcurcumin inhibits cell viability, cell invasion. Tetramethylcurcumin is a effective inhibitor of STAT3 phosphorylation, DNA-binding activity, and transactivation in vitro, leading to the impediment of multiple oncogenic processes and the induction of apoptosis in pancreatic and breast cancer cell lines[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 594.5±50.0 °C at 760 mmHg |
| Molecular Formula | C25H28O6 |
| Molecular Weight | 424.486 |
| Flash Point | 255.1±30.2 °C |
| Exact Mass | 424.188599 |
| PSA | 71.06000 |
| LogP | 4.81 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.575 |
|
~78% 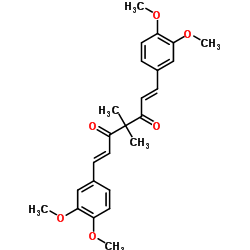
52328-97-9 |
| Literature: THE OHIO STATE UNIVERSITY RESEARCH FOUNDATION; LI, Pui-Kai; LI, Chenglong; LIN, Jiayuh; FUCHS, James, R. Patent: WO2010/121007 A1, 2010 ; Location in patent: Page/Page column 5; 14-15 ; |
|
~% 
52328-97-9 |
| Literature: Bayomi, Said M.; El-Kashef, Hassan A.; El-Ashmawy, Mahmoud B.; Nasr, Magda N. A.; El-Sherbeny, Magda A.; Badria, Farid A.; Abou-Zeid, Laila A.; Ghaly, Mariam A.; Abdel-Aziz, Naglaa I. Medicinal Chemistry Research, 2013 , vol. 22, # 3 p. 1147 - 1162 |
|
~% 
52328-97-9 |
| Literature: Bayomi, Said M.; El-Kashef, Hassan A.; El-Ashmawy, Mahmoud B.; Nasr, Magda N. A.; El-Sherbeny, Magda A.; Badria, Farid A.; Abou-Zeid, Laila A.; Ghaly, Mariam A.; Abdel-Aziz, Naglaa I. Medicinal Chemistry Research, 2013 , vol. 22, # 3 p. 1147 - 1162 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |