181695-72-7
| Name | valdecoxib |
|---|---|
| Synonyms |
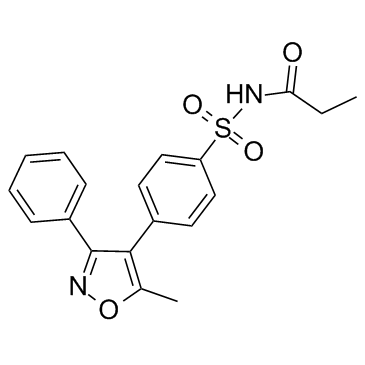
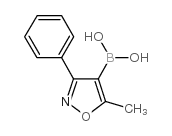
4-(5-methyl-3-phenylisoxazol-4-yl)benzensulfonamide
Bextra(Valdecoxib) 4-(4-sulfamoylphenyl)-5-methyl-3-phenyl-isoxazole T5NOJ C1 DR DSZW& ER 4-(5-Methyl-3-phenylisoxazol-4-yl)benzolsulfonamid Bextra Valdecoxib 4-[5-methyl-3-phenylisoxazol-4-yl ] benzenesulfonamide [14C]-Valdecoxib EINECS 448-010-8 Kudeq Valdyn 4-(5-Methyl-3-phenyl-1,2-oxazol-4-yl)benzenesulfonamide 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide p-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide 4-(5-Methyl-3-phenylisoxazol-4-yl)benzenesulfonamide MFCD00950568 Valdecoxib(R) |
| Description | Valdecoxib is a highly potent and selective inhibitor of COX-2, with IC50s of 5 nM and 140 μM for COX-2 and COX-1, respeceively. Valdecoxib can be used in the research of arthritis and pain. |
|---|---|
| Related Catalog | |
| Target |
COX-2:5 nM (IC50) COX-1:140 μM (IC50) |
| In Vitro | Valdecoxib (Compound 2) is a highly potent, selective and orally active inhibitor of COX-2, with IC50s of 5 nM and 140 μM for COX-2 and COX-1, respeceively[1]. Valdecoxib (10, 100 µM) inhibits LPS-induced proliferation of endothelial cells and bFGF secretion in a dose-dependent manner. Valdecoxib stimulates VEGF formation via HMEC-1 under inflammatory conditions[2]. |
| In Vivo | Valdecoxib (Compound 2) shows potent oral activity in an acute antiinflammatory assay (rat carrageenan foot pad edema; ED50 = 10.2 ± 1.4 mg/kg). Valdecoxib also has chronic antiinflammatory activity in the rat adjuvant arthritis model, with an ED50 of 0.032 ± 0.002 mg/kg/day[1]. Valdecoxib (10 mg/kg, i.p.) significantly attenuates the behavioral and biochemical (oxidative damage) alterations in chronic-stressed mice[3]. |
| Cell Assay | HMEC-1 cells proliferation is measured using the MTT conversion method. Cells are seeded (50.000 cells/well) into 96-well plates. The cells are incubated for 24 h with LPS 100 µg/mL, CoCl2 200 µM, Valdecoxib 10 or 100 µM, LPS and Valdecoxib or CoCl2 and Valdecoxib or without tested chemicals (control group). All the substances are added at the same time. After incubation, 50 µL MTT (1 mg/mL) is added and the plates are incubated at 37°C for 4 h. At the end of the experiment, cells are exposed to 100 µL DMSO, which enables the release of the blue reaction product-formazan. The absorbance at 570 nm is read on a microplate reader and results are expressed as a percentage of the absorbance measured in control cells[2]. |
| Animal Admin | Mice[3] The drugs including naproxen (14 mg/kg, i.p.), rofecoxib (5 mg/kg, i.p.), meloxicam (5 mg/kg, i.p.), nimesulide (5 mg/kg, i.p.) and Valdecoxib (10 mg/kg, i.p.) are used in the assay. The animals are randomized into 7 groups (n=10 in each group), including the naive group, in which the mice only receive vehicle for 15 d without forced swimming session; the control (chronically stressed) group, in which mice receive vehicle 30 min before the forced swimming session (6 min) for 15 d; the naproxen (14 mg/kg) group; the Valdecoxib (10 mg/kg) group; the rofecoxib (5 mg/kg) group; the meloxicam (5 mg/kg) group; and the nimesulide (5 mg/kg) group. Drugs are suspended in 0.25% carboxymethylcellulose (CMC) and administered intraperitoneally, 30 min before the forced swimming session for 15 consecutive days[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 481.2±55.0 °C at 760 mmHg |
| Melting Point | 162-164ºC |
| Molecular Formula | C16H14N2O3S |
| Molecular Weight | 314.359 |
| Flash Point | 244.8±31.5 °C |
| Exact Mass | 314.072510 |
| PSA | 94.57000 |
| LogP | 1.71 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Storage condition | Store at RT |
| Symbol |


GHS08, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H361d-H373-H410 |
| Precautionary Statements | P273-P281-P501 |
| Hazard Codes | Xn,N |
| Risk Phrases | 63-48/22-51/53 |
| Safety Phrases | 36/37-61 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2935009090 |
| Precursor 10 | |
|---|---|
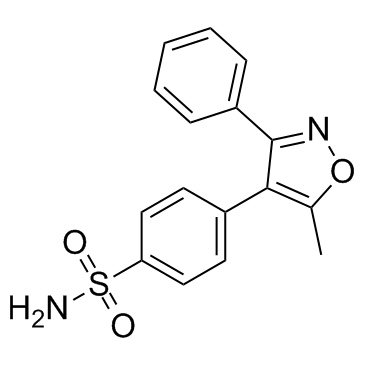
| DownStream 3 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |




![5-methyl-3-phenyl-4-(4,4,5,5-tetramethyl[1.3.2]dioxaborolan-2-yl)-isoxazole structure](https://image.chemsrc.com/caspic/404/374715-24-9.png)

![N-[[4-[5-methyl-3-phenylisoxazol-4-yl]phenyl]sulfonyl]acetamide structure](https://image.chemsrc.com/caspic/370/198471-06-6.png)