73573-88-3
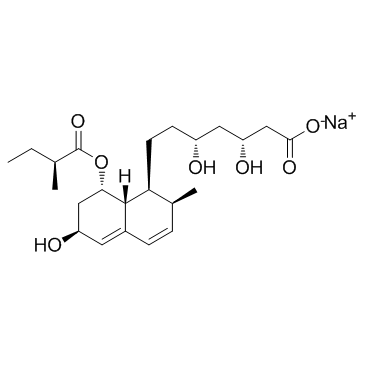
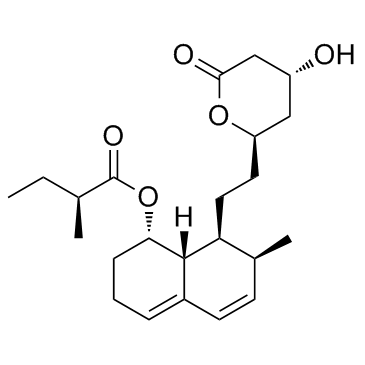
| Name | mevastatin |
|---|---|
| Synonyms |
ML 236B
Mevastatinum [INN-Latin] mevastatin Compactin (penicillium) Antibiotic ML 236B L66 AU IUTJ EOVY2&1 H1 G2- FT6OVTJ DQ &&stereoisomer Mevastatin (Compactin) MFCD05662341 Statin I [(1S,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2S)-2-methylbutanoate (+)-Compactin ML 236 B (1S,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-7-methyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl (2S)-2-methylbutanoate 2b-Methyl-8a-(2-methyl-1-oxobutoxy)mevinic acid lactone ML-236B (1S,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate 7-[1,2,6,7,8,8a-Hexahydro-2-methyl-8-(methylbutyryloxy)naphthyl]-3-hydroxyheptan-5-olide [1S-[1a(R*),7b,8b(2S*,4S*),8ab]]-2-Methylbutanoic acid 1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester Compactin Lovastatin Impurity 1 |
| Description | Mevastatin (Compactin; ML236B) inhibits HMGCR (HMG-CoA reductase) (Ki for acid form is 1 nM) which in turn inhibits isoprenoid biosynthesis and therefore blocks protein isoprenylation and reduces plasma cholesterol levels in humans. IC50 value: 1 nM (Ki)Target: HMGCRMevastatin induces apoptosis, arrests cancer cells in G1 phase and downregulates cdk 2, 4, and 6, cyclin D1 and E1, p21 and p27. Mevastatin suppresses TNF-induced NF-κB activation (IC50 = ~17 uM), which potentiates apoptosis in human myeloid leukemia cells and thus, may be useful in treating cancer. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 555.0±50.0 °C at 760 mmHg |
| Melting Point | 151-153 °C |
| Molecular Formula | C23H34O5 |
| Molecular Weight | 390.513 |
| Flash Point | 186.5±23.6 °C |
| Exact Mass | 390.240631 |
| PSA | 72.83000 |
| LogP | 3.57 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.535 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T+ |
| Risk Phrases | R26/27/28 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | EK7907100 |
| Precursor 5 | |
|---|---|
| DownStream 4 | |


![(1S,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-((2R,4R)-tetrahydro-4-methoxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthyl (2S)-2-methylbutyrate structure](https://image.chemsrc.com/caspic/220/84751-53-1.png)
![methyl 3,5-dihydroxy-7-[(1'S,2'S,8'S,8a'S)-2'methyl-8'-[(S)-2-methylbutanoyloxy]-1',2',3',7',8',8a'-hexahydro-1'-naphthyl]heptanoate structure](https://image.chemsrc.com/caspic/474/79814-60-1.png)