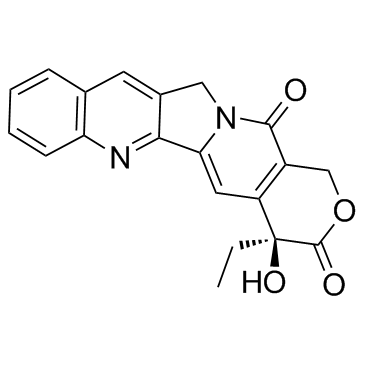
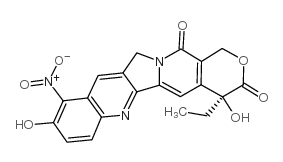
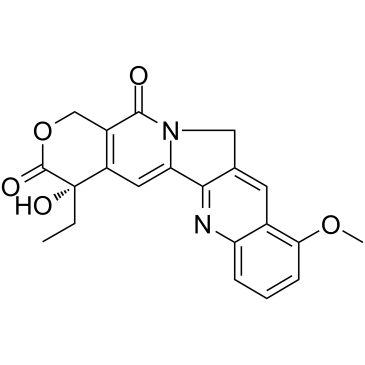
91421-42-0
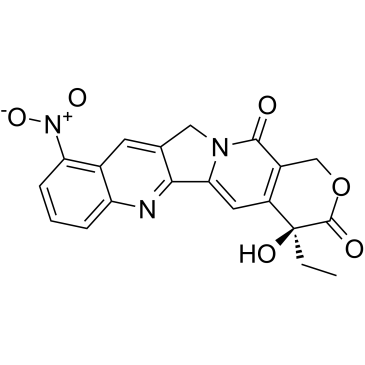
| Name | 9-Nitrocamptothecin |
|---|---|
| Synonyms |
rubitecan
MFCD06656294 PYRIDOXINE 3,4-DIPALMITATE 9-nitro-20(s)-camptothecin (4S)-4-Ethyl-4-hydroxy-10-nitro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione Orathecin |
| Description | Rubitecan (RFS 2000), a camptothecin derivative, is an orally active topoisomerase I inhibitor with broad antitumor activity, and induces protein-linked DNA single-strand breaks, thereby blocking DNA and RNA synthesis in dividing cells[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I[1] |
| In Vitro | Rubitecan (RFS 2000) inhibits U-CH1, U-CH2, and CCL4 cells with IC50s 0.32, 0.83, and 7.7 µM, respectively[4]. |
| References |
[3]. Rubitecan |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 816.3±65.0 °C at 760 mmHg |
| Melting Point | 182-186ºC |
| Molecular Formula | C20H15N3O6 |
| Molecular Weight | 393.350 |
| Flash Point | 447.5±34.3 °C |
| Exact Mass | 393.096100 |
| PSA | 127.24000 |
| LogP | 1.38 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.762 |
| Storage condition | Room temp |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | UQ0493300 |
| Precursor 6 | |
|---|---|
| DownStream 4 | |


![(S)-4-ethyl-4-hydroxy-10-nitro-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 4-nitrobenzenesulfonate structure](https://image.chemsrc.com/caspic/085/172917-96-3.png)
![(S)-4-ethyl-4-hydroxy-10-nitro-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl benzenesulfonate structure](https://image.chemsrc.com/caspic/161/172917-94-1.png)
![(S)-4-ethyl-4-hydroxy-10-nitro-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 4-fluorobenzenesulfonate structure](https://image.chemsrc.com/caspic/391/164159-91-5.png)