Fmoc-L-Lys(4-N3-Z)-OH
Modify Date: 2025-09-21 09:06:37
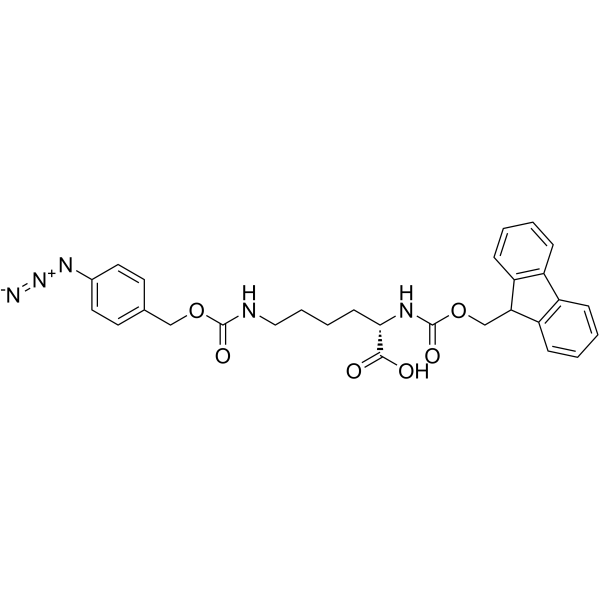
Fmoc-L-Lys(4-N3-Z)-OH structure
|
Common Name | Fmoc-L-Lys(4-N3-Z)-OH | ||
|---|---|---|---|---|
| CAS Number | 1446511-14-3 | Molecular Weight | 543.57 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C29H29N5O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Fmoc-L-Lys(4-N3-Z)-OHFmoc-L-Lys(4-N3-Z)-OH is a click chemistry reagent containing an azide group. Fmoc-L-Lys(4-N3-Z)-OH acts as Lysine building-block for SPPS containing an Azide moiety as a bioorthogonal ligation handle, an infrared probe and a photo-affinity reagent. It can be decaged by trans-cyclooctenols via a strain-promoted 1,3-dipolar cycloaddition[1][2]. |
| Name | Fmoc-L-Lys(4-N3-Z)-OH |
|---|
| Description | Fmoc-L-Lys(4-N3-Z)-OH is a click chemistry reagent containing an azide group. Fmoc-L-Lys(4-N3-Z)-OH acts as Lysine building-block for SPPS containing an Azide moiety as a bioorthogonal ligation handle, an infrared probe and a photo-affinity reagent. It can be decaged by trans-cyclooctenols via a strain-promoted 1,3-dipolar cycloaddition[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C29H29N5O6 |
|---|---|
| Molecular Weight | 543.57 |